Pregnancy and Infant Outcomes in Women With Multiple Sclerosis Treated With Ocrelizumab
- PMID: 39689270
- PMCID: PMC11655168
- DOI: 10.1212/NXI.0000000000200349
Pregnancy and Infant Outcomes in Women With Multiple Sclerosis Treated With Ocrelizumab
Abstract
Background and objectives: Ocrelizumab labeling advises contraception for women during treatment and for 6-12 months thereafter. Because pregnancies may occur during this time, it is critical to understand pregnancy and infant outcomes in women with multiple sclerosis (MS) after ocrelizumab exposure.
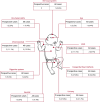
Methods: Pregnancy cases reported to Roche global pharmacovigilance until 12 July 2023 were analyzed. In utero exposure was defined if the last ocrelizumab infusion occurred in the 3 months before the last menstrual period or during pregnancy. Breastfeeding exposure was defined if at least one infusion occurred while breastfeeding. Fetal death was termed spontaneous abortion (SA) if < 22 complete gestational weeks (GWs) and stillbirth if later. Live births (LBs) were preterm if < 37 complete GWs. Major congenital anomalies (MCAs), infant outcomes, and maternal complications were also analyzed.
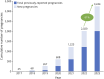
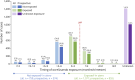
Results: In total, 3,244 pregnancies were reported in women with MS receiving ocrelizumab. The median maternal age was 32 years (Q1-Q3: 29-35 years), and most women had relapsing MS (65.6%). Of 2,444 prospectively reported pregnancies, 855 were exposed to ocrelizumab in utero (512 with a known outcome), 574 were nonexposed, and the remaining 1,015 had unknown timing of exposure. Most (83.6%; 956/1,144) of the pregnancies with a known outcome resulted in LBs (exposed, 84.2%; nonexposed, 88.3%). The exposed and nonexposed groups had similar proportions of other important pregnancy outcomes (preterm births, 9.5% vs 8.7%; SA, 7.4% vs 9.1%). Elective abortions were more frequent in the exposed group (7.4%, vs 1.7% in the nonexposed group). The proportion of LBs with MCAs was similar between the exposed and nonexposed groups (2.1% vs 1.9%) and within epidemiologic background rates. In the exposed group, one stillbirth and one neonatal death were prospectively reported.
Discussion: In this analysis of a large pregnancy outcome dataset for an anti-CD20 in MS, in utero exposure to ocrelizumab was not associated with an increased risk of adverse pregnancy or infant outcomes. These data will enable neurologists and women with MS to make more informed decisions around family planning, balancing safety risks to the fetus/infant against the importance of disease control in the mother.
Conflict of interest statement
S Vukusic received grants and research support from Biogen, Novartis, Merck-Serono, F. Hoffmann-La Roche Ltd and Sanofi-Genzyme; consulting fees from F. Hoffmann-La Roche Ltd, Biogen, BMS-Celgene, Janssen, Novartis, Merck-Serono, Sandoz, Sanofi-Genzyme and Teva; and payment/honoraria for lectures, speaking etc. from F. Hoffmann-La Roche Ltd, Biogen, BMS-Celgene, Novartis, Merck-Serono, Sandoz, Sanofi-Genzyme and Teva. R Bove received research support from the National Institutes of Health, National Multiple Sclerosis Society, Hilton Foundation, California Initiative to Advance Precision Medicine, Tom Sherak Foundation, Biogen, Novartis and F. Hoffmann-La Roche Ltd/Genentech; and personal compensation for consulting from Alexion, Biogen, EMDSerono, Novartis, Sanofi-Genzyme, F. Hoffmann-La Roche Ltd/Genentech and TG Therapeutics. R Dobson received research support from Multiple Sclerosis Society UK, Horne Family Foundation, Barts Charity, Merck, Biogen and Celgene; consulting fees from F. Hoffmann-La Roche Ltd, Novartis, Janssen and Biogen (all payments made are institutional and used to support research/educational activities); honoraria for lectures, speaking etc. from Biogen, F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck, Novartis, Janssen and Teva; and support for attending meetings and/or travel from Novartis, Biogen and Janssen (all payments made are institutional and used to support research/educational activities) and is a member of the Association of British Neurologists MS Advisory Group and NHSE Neurology CAG. T McElrath received research support from the National Institutes of Health and NX Prenatal Inc.; compensation for service on the scientific advisory boards of NX Prenatal Inc., Mirvie Inc., F. Hoffmann-La Roche Ltd.; and consulting fees from F. Hoffmann-La Roche Ltd and Comanche Biopharma. C Oreja-Guevara received honoraria for consulting and serving on advisory boards from Biogen Idec., F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck, Novartis, BMS, Viatris, Janssen, Alexion, Horizon, and Teva Genzyme, Merck, Novartis and Teva. C Pietrasanta received consulting fees from F. Hoffmann-La Roche Ltd. C-J Lin is an employee of and shareholder in F. Hoffmann-La Roche Ltd. G Ferreira is a consultant for F. Hoffmann-La Roche Ltd. L Craveiro is an employee of and shareholder in F. Hoffmann-La Roche Ltd. D Zecevic is an employee of and shareholder in F. Hoffmann-La Roche Ltd. N Pasquarelli is an employee of and shareholder in F. Hoffmann-La Roche Ltd. K Hellwig received grant/contract support, consulting fees, honoraria and/or compensation from the Federal Innovationsfonds, National MS Society in Germany, Almiral Bayer, Biogen, Sanofi, Teva, F. Hoffmann-La Roche Ltd, Novartis and Merck. Go to
Figures
References
-
- Portaccio E, Tudisco L, Pastò L, et al. ; MS Study Group of the Italian Neurological Society. Pregnancy in multiple sclerosis women with relapses in the year before conception increases the risk of long-term disability worsening. Mult Scler. 2022;28(3):472-479. doi: 10.1177/13524585211023365 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
