Porphyromonas gingivalis outer membrane vesicles increase vascular permeability by inducing stress fiber formation and degrading vascular endothelial-cadherin in endothelial cells
- PMID: 39690116
- PMCID: PMC11970716
- DOI: 10.1111/febs.17349
Porphyromonas gingivalis outer membrane vesicles increase vascular permeability by inducing stress fiber formation and degrading vascular endothelial-cadherin in endothelial cells
Abstract
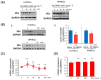
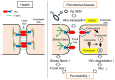
Porphyromonas gingivalis (Pg) is a keystone bacterium associated with systemic diseases, such as diabetes mellitus and Alzheimer's disease. Outer membrane vesicles (OMVs) released from Pg have been implicated in systemic diseases by delivering Pg virulence factors to host cells in distant organs and inducing cellular dysfunction. Pg OMVs also have the potential to enter distant organs via the bloodstream. However, the effects of Pg OMVs on the vascular function are poorly understood. Here, we showed that Pg OMVs increase vascular permeability by promoting stress fiber formation and lysosome/endosome-mediated vascular endothelial-cadherin (VEc) degradation in human umbilical vein endothelial cells (HUVECs) and human pulmonary microvascular endothelial cells (HPMECs). F-actin, visualized via fluorescein isothiocyanate-phalloidin, became thicker and longer, leading to the formation of radical stress fibers in response to Pg OMVs in HUVECs and HPMECs. Western blotting and quantitative real-time polymerase chain reaction analyses revealed that Pg OMVs decreased VEc protein levels in a gene-independent manner. Pg OMVs enhanced vesicular VEc accumulation in the cytoplasm around lysosome-associated membrane protein 1-positive structures during pretreatment with the lysosomal inhibitor chloroquine. This suggests that Pg OMVs decrease VEc protein levels by accelerating their internalization and degradation via lysosomes and endosomes. A27632 inhibition of Rho kinases impaired the Pg OMV-induced stress fiber formation and VEc degradation, resulting in the recovery of hyperpermeability. These findings provide new insights into the pathogenesis of systemic diseases that are associated with periodontal diseases.
Keywords: Porphyromonas gingivalis; outer membrane vesicles; periodontal diseases; vascular permeability.
© 2024 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Watanabe K, Katagiri S, Takahashi H, Sasaki N, Maekawa S, Komazaki R, Hatasa M, Kitajima Y, Maruyama Y, Shiba T et al. (2021) Porphyromonas gingivalis impairs glucose uptake in skeletal muscle associated with altering gut microbiota. FASEB J 35, e21171. - PubMed
-
- Ishikawa M, Yoshida K, Okamura H, Ochiai K, Takamura H, Fujiwara N & Ozaki K (2013) Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK‐3beta signaling pathway. Biochim Biophys Acta 1832, 2035–2043. - PubMed
-
- Veith PD, Chen YY, Gorasia DG, Chen D, Glew MD, O'Brien‐Simpson NM, Cecil JD, Holden JA & Reynolds EC (2014) Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res 13, 2420–2432. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

