Faecal immunochemical tests for patients with symptoms suggestive of colorectal cancer: An updated systematic review and multiple-threshold meta-analysis of diagnostic test accuracy studies
- PMID: 39690130
- PMCID: PMC11683176
- DOI: 10.1111/codi.17255
Faecal immunochemical tests for patients with symptoms suggestive of colorectal cancer: An updated systematic review and multiple-threshold meta-analysis of diagnostic test accuracy studies
Abstract
Aim: Extending faecal immunochemical tests for haemoglobin (FIT) to all primary care patients with symptoms suggestive of colorectal cancer (CRC) could identify people who are likely to benefit from colonoscopy and facilitate earlier treatment. The aim of this work was to investigate the diagnostic accuracy of FIT across different analysers at different thresholds, as a single test or in duplicate (dual FIT).
Method: This systematic review and meta-analysis searched 10 sources (December 2022). Diagnostic accuracy studies of HM-JACKarc, OC-Sensor, FOB Gold, QuikRead go, NS-Prime and four Immunodiagnostik (IDK) tests in primary care patients were included. Risk of bias was assessed (QUADAS-2). Statistical syntheses produced summary estimates of sensitivity and specificity at any chosen threshold for CRC, inflammatory bowel disease and advanced adenomas separately. Sensitivity analyses investigated reference standard and population type (high, low or all-risk). Subgroup analyses investigated patient characteristics (e.g. anaemia, age, sex, ethnicity).
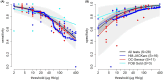
Results: Thirty-seven studies were included. At a threshold of 10 μg/g, pooled results for sensitivity and specificity (95% credible intervals) for CRC, respectively, were: HM-JACKarc (n = 16 studies) 89.5% (84.6%-93.4%) and 82.8% (75.2%-89.6%); OC-Sensor (n = 11 studies) 89.8% (85.9%-93.3%) and 77.6% (64.3%-88.6%); FOB Gold (n = 3 studies), 87.0% (67.3%-98.3%) and 88.4% (81.7%-94.2%). There were limited or no data on the other tests, dual FIT and relating to patient characteristics.
Conclusion: Test sensitivity at a threshold of 10 μg/g highlights a requirement for adequate safeguards in test-negative patients with ongoing symptoms. Further research is needed into the impact of patient characteristics and dual FIT.
Keywords: adenomas; colorectal cancer; diagnostic test accuracy; faecal immunochemical test; inflammatory bowel disease; primary care; systematic review.
© 2024 The Author(s). Colorectal Disease published by John Wiley & Sons Ltd on behalf of Association of Coloproctology of Great Britain and Ireland.
Conflict of interest statement
None of the authors have any financial conflicts of interest to declare. Muti Abulafi, Alex Ball, Sally Benton, Richard Booth, Rachel Carten, Willie Hamilton, Matt Kurien and Kevin Monahan were all authors on studies included in the review or on the review we updated.
Figures

References
-
- Monahan KJ, Davies MM, Abulafi M, Banerjea A, Nicholson BD, Arasaradnam R, et al. Faecal immunochemical testing (FIT) in patients with signs or symptoms of suspected colorectal cancer (CRC): a joint guideline from the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the British Society of Gastroenterology (BSG). Gut. 2022;12:1962. - PMC - PubMed
-
- Booth R, Carten R, D'Souza N, Westwood M, Kleijnen J, Abulafi M. Role of the faecal immunochemical test in patients with risk‐stratified suspected colorectal cancer symptoms: a systematic review and meta‐analysis to inform the ACPGBI/BSG guidelines. Lancet Reg Health Eur. 2022;23:100518. - PMC - PubMed
-
- NICE . Quantitative faecal immunochemical tests to guide colorectal cancer pathway referral in primary care. Final scope. 2022.
-
- The Cochrane . Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). 2019.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

