Macrophages promote pre-metastatic niche formation of breast cancer through aryl hydrocarbon receptor activity
- PMID: 39690159
- PMCID: PMC11652640
- DOI: 10.1038/s41392-024-02042-5
Macrophages promote pre-metastatic niche formation of breast cancer through aryl hydrocarbon receptor activity
Abstract
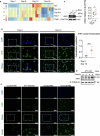
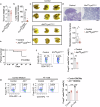
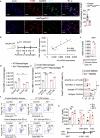
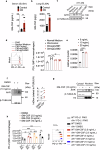
Macrophages that acquire an immunosuppressive phenotype play a crucial role in establishing the pre-metastatic niche (PMN), which is essential for facilitating breast cancer metastasis to distant organs. Our study showed that increased activity of the aryl hydrocarbon receptor (AHR) in lung macrophages plays a crucial role in establishing the immunosuppressive PMN in breast cancer. Specifically, AHR activation led to high expression of PD-L1 on macrophages by directly binding to the promoter of Pdl1. This upregulation of PD-L1 promoted the differentiation of regulatory T cells (Tregs) within the PMN, further enhancing immunosuppressive conditions. Mice with Ahr conditional deletion in macrophages had reduced lung metastasis of breast cancer. The elevated AHR levels in PMN macrophages were induced by GM-CSF, which was secreted by breast cancer cells. Mechanistically, the activated STAT5 signaling pathway induced by GM-CSF prevented AHR from being ubiquitinated, thereby sustaining its activity in macrophages. In breast cancer patients, the expression of AHR and PD-L1 was correlated with increased Treg cell infiltration, and higher levels of AHR were associated with a poor prognosis. These findings reveal that the crosstalk of breast cancer cells, lung macrophages, and Treg cells via the GM-CSF-STAT5-AHR-PD-L1 cascade modulates the lung pre-metastatic niche during breast cancer progression.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- 82430086, 81930085/National Natural Science Foundation of China (National Science Foundation of China)
- 82425032, 32070872, 31771641/National Natural Science Foundation of China (National Science Foundation of China)
- 82301571/National Natural Science Foundation of China (National Science Foundation of China)
- 82470658/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

