A prospective, multicentre study evaluating safety and efficacy of a fixed dose combination of Remogliflozin etabonate, Vildagliptin, and Metformin in Indian patients with type 2 diabetes mellitus (Triad-RMV)
- PMID: 39690416
- PMCID: PMC11654189
- DOI: 10.1186/s40842-024-00210-8
A prospective, multicentre study evaluating safety and efficacy of a fixed dose combination of Remogliflozin etabonate, Vildagliptin, and Metformin in Indian patients with type 2 diabetes mellitus (Triad-RMV)
Abstract
Aims: The ICMR INDIAB-17 study revealed a diabetes prevalence of 11.4% in India, emphasizing the need for effective treatment for glycemic control. A Phase IV study was conducted to evaluate the safety and efficacy of a Fixed Dose Combination (FDC) of Remogliflozin, Metformin and Vildagliptin (RMV) in Type 2 Diabetes Mellitus (T2DM) patients uncontrolled on Metformin plus SGLT2 inhibitor or Metformin plus DPP4 inhibitor dual therapy.
Methods: A total of 215 patients (mean age: 46.4 years; 64% male, 36% female) were enrolled across multiple centers in India. The study population included patients with a baseline HbA1c ≥ 8% at the time of screening. The primary objective was to assess safety based on treatment-emergent adverse events (TEAEs), while the secondary. aim was to evaluate effectiveness in terms of glycemic (HbA1c, fasting plasma glucose, postprandial glucose) and extra-glycemic measures (renal and lipid parameters). Statistical analysis was conducted using paired t-tests and the Wilcoxon signed-rank test for within-group comparisons, and the Bonferroni correction was applied to adjust for multiple comparisons. Effectiveness was evaluated at baseline, week 12, and week 24.
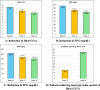
Results: The study demonstrated statistically significant reductions in mean HbA1c levels from baseline to both week 12 and week 24 (p < 0.00001). At 24, weeks, 45.1% of patients achieved target HbA1c levels of ≤ 7%. Significant reduction was also observed in fasting plasma glucose (FPG) and postprandial glucose (PPG) levels. Renal parameters remained stable or improved, and lipid profile parameters, including LDL-C and triglycerides, showed favorable changes. Adverse events of special interest, including hypoglycemia and urinary tract infections, were reported in 4.7% of patients, with no serious adverse event recorded.
Conclusions: The twice daily triple FDC of RMV was well tolerated, safe and effective in patients with Type 2 Diabetes Mellitus uncontrolled on dual drug therapy of Metformin plus SGLT2i or Metformin plus DPP4i. The treatment led to significant improvements in glycemic control and other metabolic parameters over 24 weeks, without compromising renal function or causing serious adverse events.
Trial registration: CTRI, CTRI/2022/05/042581. Registered 17 May 2022, https//ctri.nic.in/Clinicaltrials/rmaindet.php? trialid=68,757&EncHid=36127.16500&modid=1&compid=19.
Keywords: Fixed dose combination; Indian study; Metformin; Remogliflozin; Type 2 diabetes; Vildagliptin.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The work presented in this study was in accordance with the study protocol, the New Drugs and Clinical Trials Rules 2019 issued by the Government of India, the ethical principles that have their origin in the Declaration of Helsinki, International Council for Harmonisation (ICH) Good Clinical Practice (GCP), and all applicable local regulatory requirements. Informed consent was obtained from all the study subjects who took part in the trial. Ethic committee approval was obtained from all respective sites and study was registered in CTRI (CTRI/2022/05/042581). Consent for publication: We, hereby transfer all copyright ownership of the manuscript entitled " A Prospective, Multicentre Study Evaluating Safety and Efficacy of a Fixed Dose Combination of Remogliflozin Etabonate, Vildagliptin, and Metformin in Indian Patients with Type 2 Diabetes Mellitus (Triad-RMV)” to ‘Clinical Diabetes and Endocrinology’ effective from the date of submission. We affirm that the manuscript is original, that we are the authors of the manuscript, and that it has not been published previously nor is it under consideration for publication elsewhere. We also affirm that the manuscript does not infringe upon any existing copyright or any other rights of any third party and that we have obtained all necessary permissions for any third-party materials included in the manuscript. Competing interests: Corresponding author has received funding from Glenmark Pharmaceuticals Ltd for research and its publication. The other authors declare that they have no competing interests.
Figures



References
-
- Anjana RM, Unnikrishnan R, Deepa M, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diab Endocrinol. 2023;11(7):474–89. 10.1016/s2213-8587(23)00119-5. - PubMed
-
- Sahay RK, Giri R, Shembalkar JV, et al. Fixed-dose combination of dapagliflozin + sitagliptin + metformin in patients with type 2 diabetes poorly controlled with metformin: phase 3, randomized comparison with dual combinations. Adv Ther. 2023;40(7):3227–46. 10.1007/s12325-023-02523-z. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

