Role of PD-1/PD-L1 signaling axis in oncogenesis and its targeting by bioactive natural compounds for cancer immunotherapy
- PMID: 39690423
- PMCID: PMC11654217
- DOI: 10.1186/s40779-024-00586-9
Role of PD-1/PD-L1 signaling axis in oncogenesis and its targeting by bioactive natural compounds for cancer immunotherapy
Abstract
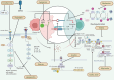
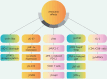
Cancer is a global health problem and one of the leading causes of mortality. Immune checkpoint inhibitors have revolutionized the field of oncology, emerging as a powerful treatment strategy. A key pathway that has garnered considerable attention is programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1). The interaction between PD-L1 expressed on tumor cells and PD-1 reduces the innate immune response and thus compromises the capability of the body's immune system. Furthermore, it controls the phenotype and functionality of innate and adaptive immune components. A range of monoclonal antibodies, including avelumab, atezolizumab, camrelizumab, dostarlimab, durvalumab, sinitilimab, toripalimab, and zimberelimab, have been developed for targeting the interaction between PD-1 and PD-L1. These agents can induce a broad spectrum of autoimmune-like complications that may affect any organ system. Recent studies have focused on the effect of various natural compounds that inhibit immune checkpoints. This could contribute to the existing arsenal of anticancer drugs. Several bioactive natural agents have been shown to affect the PD-1/PD-L1 signaling axis, promoting tumor cell apoptosis, influencing cell proliferation, and eventually leading to tumor cell death and inhibiting cancer progression. However, there is a substantial knowledge gap regarding the role of different natural compounds targeting PD-1 in the context of cancer. Hence, this review aims to provide a common connection between PD-1/PD-L1 blockade and the anticancer effects of distinct natural molecules. Moreover, the primary focus will be on the underlying mechanism of action as well as the clinical efficacy of bioactive molecules. Current challenges along with the scope of future research directions targeting PD-1/PD-L1 interactions through natural substances are also discussed.
Keywords: Cancer; Crosstalk; Natural compounds; PD-1/PD-L1; Therapeutic targets.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
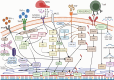
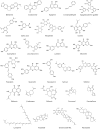
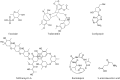
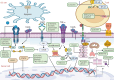
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

