Association of Synovial Innate Immune Exhaustion With Worse Pain in Knee Osteoarthritis
- PMID: 39690716
- PMCID: PMC12123257
- DOI: 10.1002/art.43089
Association of Synovial Innate Immune Exhaustion With Worse Pain in Knee Osteoarthritis
Abstract
Objective: Uncontrolled pain remains a major clinical challenge in the management of knee osteoarthritis (OA), the most common disabling joint disease. Worse pain is associated with synovial innate immune cell infiltration (synovitis), but the role of innate immune-regulatory cells in pain is unknown. Our objective was to identify synovial innate immune cell subsets and pathophysiologic mechanisms associated with worse pain in patients with knee OA.
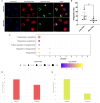
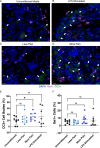
Methods: Synovial tissue biopsies from 122 patients with mild-to-severe knee OA pain (Knee Injury and OA Outcome Score [KOOS]) were analyzed to identify associations between synovial histopathology and worse pain. We then used spatial transcriptomics and proteomics of synovial tissue microenvironments (n = 32), followed by single-cell RNA sequencing (n = 8), to identify synovial cell composition and cell-cell communication networks in patients with more severe OA pain.
Results: Histopathological signs of synovial microvascular dysfunction and perivascular edema were associated with worse KOOS pain (-10.76; 95% confidence interval [CI] -18.90 to -2.61). Patients with worse pain had fewer immune-regulatory macrophages, expanded fibroblast subsets, and enrichment in neurovascular remodeling pathways. Synovial macrophages from patients with worse pain expressed markers of immune exhaustion and decreased phagocytic function (-19.42%; 95% CI -35.96 to -2.89) and their conditioned media increased neuronal cell stress in dorsal root ganglia.
Conclusion: Although synovitis increases during OA, our findings suggest that exhaustion, dysfunction, and loss of immune-regulatory macrophages is associated with worse pain and may be an important therapeutic target.
© 2024 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures


References
-
- Oo WM, Linklater JM, Bennell KL, et al. Are OMERACT knee osteoarthritis ultrasound scores associated with pain severity, other symptoms, and radiographic and magnetic resonance imaging findings? J Rheumatol 2021;48(2):270–278. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

