Investigating Task-Free Functional Connectivity Patterns in Newborns Using Functional Near-Infrared Spectroscopy
- PMID: 39690863
- PMCID: PMC11652786
- DOI: 10.1002/brb3.70180
Investigating Task-Free Functional Connectivity Patterns in Newborns Using Functional Near-Infrared Spectroscopy
Abstract
Background: Resting-state networks (RSNs), particularly the sensorimotor network, begin to strengthe in the third trimester of pregnancy and mature extensively by term age. The integrity and structure of these networks have been repeatedly linked to neurological health outcomes in neonates, highlighting the importance of understanding the normative variations in RSNs in healthy development. Specifically, robust bilateral functional connectivity in the sensorimotor RSN has been linked to optimal neurodevelopmental outcomes in neonates.
Aim: In the current study, we aimed to map the developmental trajectory of the sensorimotor RSN in awake neonates using functional near-infrared spectroscopy (fNIRS).
Materials & methods: We acquired fNIRS resting-state data from 41 healthy newborns (17 females, gestational age ranging from 36 + 0 to 42 + 1 weeks) within the first week after birth. We performed both single channel and hemispheric analyses to investigate the relationship between functional connectivity and both gestational and postnatal age.
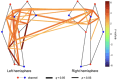
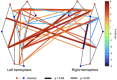
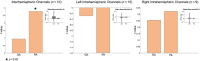
Results: We observed robust positive connectivity in numerous channel-pairs across the sensorimotor network, especially in the left hemisphere. Next, we examined the relationship between functional connectivity, gestational age, and postnatal age, while controlling for sex and subject effects. We found both gestational and postnatal age to be significantly associated with changes in functional connectivity in the sensorimotor RSN. In our hemispheric analysis (Ninterhemispheric = 10, Nleft intrahemispheric = 15, and Nright intrahemispheric = 9), we observed a significant positive relationship between interhemispheric connectivity and postnatal age.
Discussion and conclusion: In summary, our findings demonstrate the utility of fNIRS for monitoring early developmental changes in functional networks in awake newborns.
Keywords: connectivity; development; fNIRS; newborn.
© 2024 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Bulgarelli, C. , Blasi A., McCann S., et al. 2024. “Growth in Early Infancy Drives Optimal Brain Functional Connectivity Which Predicts Cognitive Flexibility in Later Childhood.” eLife 13: RP94194. 10.7554/eLife.94194.1. - DOI
-
- Chorna, O. , Solomon J. E., Slaughter J. C., Stark A. R., and Maitre N. L.. 2014. “Abnormal Sensory Reactivity in Preterm Infants During the First Year Correlates With Adverse Neurodevelopmental Outcomes at 2 Years of Age.” Archives of Disease in Childhood—Fetal and Neonatal Edition 99, no. 6: F475–F479. 10.1136/archdischild-2014-306486. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

