In vitro hair growth-promoting effects of araliadiol via the p38/PPAR-γ signaling pathway in human hair follicle stem cells and dermal papilla cells
- PMID: 39691387
- PMCID: PMC11649413
- DOI: 10.3389/fphar.2024.1482898
In vitro hair growth-promoting effects of araliadiol via the p38/PPAR-γ signaling pathway in human hair follicle stem cells and dermal papilla cells
Abstract
Background: Scalp hair plays a crucial role in social communication by expressing personal appearance and self-identity. Consequently, hair loss often leads to a perception of unattractiveness, negatively impacting an individual's life and mental health. Currently, the use of Food and Drug Administration (FDA)-approved drugs for hair loss is associated with several side effects, highlighting the need for identifying new drug candidates, such as plant-derived phytochemicals, to overcome these issues.
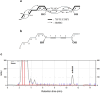
Objective: This study investigated the hair growth-promoting effects of araliadiol, a polyacetylene compound found in plants such as Centella asiatica.
Methods: We employed an in vitro model comprising human hair follicle stem cells (HHFSCs) and human dermal papilla cells (HDPCs) to evaluate the hair growth-promoting effects of araliadiol. The proliferation-stimulating effects of araliadiol were assessed using water-soluble tetrazolium salt assay, adenosine triphosphate content assay, and crystal violet staining assay. In addition, we performed luciferase reporter assay, polymerase chain reaction analysis, cell fractionation, Western blot analysis, and enzyme-linked immunosorbent assay (ELISA) to elucidate the mechanism underlying the hair growth-inductive effects of araliadiol.
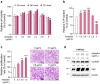
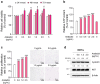
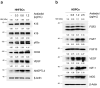
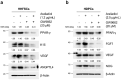
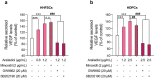
Results: Araliadiol exhibited both proliferation- and hair growth-promoting effects in HHFSCs and HDPCs. Specifically, it increased the protein expression of cyclin B1 and Ki67. In HHFSCs, it elevated the expression of hair growth-promoting factors, including CD34, vascular endothelial growth factor (VEGF), and angiopoietin-like 4. Similarly, araliadiol increased the expression of hair growth-inductive proteins such as fibroblast growth factor 7, VEGF, noggin, and insulin-like growth factor 1 in HDPCs. Subsequent Western blot analysis and ELISA using inhibitors such as GW9662 and SB202190 confirmed that these hair growth-promoting effects were dependent on the p38/PPAR-γ signaling in both HHFSCs and HDPCs.
Conclusion: Araliadiol promotes hair growth through the p38/PPAR-γ signaling pathway in human hair follicle cells. Therefore, araliadiol can be considered a novel drug candidate for the treatment of alopecia.
Keywords: PPAR-γ; alopecia; araliadiol; hair follicles; hair growth; hair loss; phytochemicals; polyacetylene.
Copyright © 2024 Park, Park, Seo, Yoo and Bae.
Conflict of interest statement
Authors HP, DS, and DY were employed by ASK Company Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The Effect of JAK Inhibitor on the Survival, Anagen Re-Entry, and Hair Follicle Immune Privilege Restoration in Human Dermal Papilla Cells.Int J Mol Sci. 2020 Jul 20;21(14):5137. doi: 10.3390/ijms21145137. Int J Mol Sci. 2020. PMID: 32698510 Free PMC article.
-
Injectable platelet rich fibrin facilitates hair follicle regeneration by promoting human dermal papilla cell proliferation, migration, and trichogenic inductivity.Exp Cell Res. 2021 Dec 1;409(1):112888. doi: 10.1016/j.yexcr.2021.112888. Epub 2021 Oct 26. Exp Cell Res. 2021. PMID: 34715152
-
Effect of Plantago asiatica L. extract on the anagen phase in human hair follicle dermal papilla cells.J Cosmet Dermatol. 2023 Aug;22(8):2324-2332. doi: 10.1111/jocd.15720. Epub 2023 Mar 31. J Cosmet Dermatol. 2023. PMID: 36999450
-
Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature.Regen Ther. 2022 Oct 31;21:527-539. doi: 10.1016/j.reth.2022.10.005. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36382136 Free PMC article. Review.
-
PPAR-γ signalling as a key mediator of human hair follicle physiology and pathology.Exp Dermatol. 2020 Mar;29(3):312-321. doi: 10.1111/exd.14062. Epub 2019 Dec 17. Exp Dermatol. 2020. PMID: 31769892 Review.
Cited by
-
Pharmacological Evaluation of Araliadiol as a Novel Anti-Inflammatory Agent in LPS-Induced RAW 264.7 Cells.Biomedicines. 2025 Jun 8;13(6):1408. doi: 10.3390/biomedicines13061408. Biomedicines. 2025. PMID: 40564127 Free PMC article.
References
-
- Abdullah A. (2020). Comparative study of the online over-the-counter hair loss products. J. Dermatology Res. Ther. 6. 10.23937/2469-5750/1510077 - DOI
-
- Ahmed M. H., Karkush S. I., Ali S. A., Mohammed A. A. (2024). Phytochemicals: a new arsenal in drug discovery. Int. J. Med. Sci. Dent. Health 10 (01), 29–44. 10.55640/ijmsdh-10-01-03 - DOI
-
- Asnaashari S., Javadzadeh Y. (2020). Herbal medicines for treatment of androgenic alopecia. Altern. Ther. Health and Med. 26 (4), 27–35. - PubMed
LinkOut - more resources
Full Text Sources

