Conceptual basis for the development of guidance for the use of biomarkers of effect in regulatory risk assessment of chemicals
- PMID: 39691503
- PMCID: PMC11650060
- DOI: 10.2903/j.efsa.2024.9153
Conceptual basis for the development of guidance for the use of biomarkers of effect in regulatory risk assessment of chemicals
Abstract
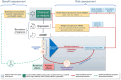
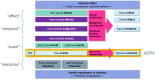
This Scientific Report was carried out in the context of the self-task mandate (M-2023-00097) of the EFSA's Scientific Committee on 'Guidance on the use of biomarkers of effect in regulatory risk assessment of chemicals'. In the first phase, the project on biomarkers of effect started with a feasibility study (EFSA-Q-2024-00128), with the intention to look closer at definitions and descriptions of biomarkers of effect, as well as to explore several concepts related to the context of application and other scientific principles to be further considered for its development. In addition, relevant activities, initiatives and knowledge in this area were collected and analysed within a complementary mapping study. The outcome of this phase aimed to create a structured basis for future guidance, to identify challenges and to recommend a way forward for its development. The recommendations refer especially to terminologies, the scope of the guidance and several scientific and technical aspects of the selection and interpretation of biomarkers of effect that need to be addressed in future guidance. Moreover, further recommendation refers to the collaborative process to be established with other regulatory organisations that should support the harmonisation and reduce divergencies in the application of methodologies across organisations or sectors.
Keywords: adverse effect; adverse outcome pathways; biomarkers; biomarkers of effect; risk assessment.
© 2024 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Figures

Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
The principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment.EFSA J. 2018 Jan 24;16(1):e05122. doi: 10.2903/j.efsa.2018.5122. eCollection 2018 Jan. EFSA J. 2018. PMID: 32625670 Free PMC article.
-
Scientific motivations and criteria to consider updating EFSA scientific assessments.EFSA J. 2017 Mar 17;15(3):e04737. doi: 10.2903/j.efsa.2017.4737. eCollection 2017 Mar. EFSA J. 2017. PMID: 32625443 Free PMC article.
-
Translating data into policy informing decisions: current and future perspectives from the European Food Safety Authority (EFSA).Proc Nutr Soc. 2025 Feb 5:1-9. doi: 10.1017/S0029665125000047. Online ahead of print. Proc Nutr Soc. 2025. PMID: 39905747 Review.
-
Risk Assessment of Combined Exposure to Multiple Chemicals at the European Food Safety Authority: Principles, Guidance Documents, Applications and Future Challenges.Toxins (Basel). 2023 Jan 4;15(1):40. doi: 10.3390/toxins15010040. Toxins (Basel). 2023. PMID: 36668860 Free PMC article. Review.
Cited by
-
Molecular response of Chironomus riparius to antibiotics.Curr Res Toxicol. 2025 May 18;8:100239. doi: 10.1016/j.crtox.2025.100239. eCollection 2025. Curr Res Toxicol. 2025. PMID: 40491715 Free PMC article.
References
-
- Baken, K. A. , Lambrechts, N. , Remy, S. , Mustieles, V. , Rodríguez‐Carrillo, A. , Neophytou, C. M. , Olea, N. , & Schoeters, G. (2019). A strategy to validate a selection of human effect biomarkers using adverse outcome pathways: Proof of concept for phthalates and reproductive effects. Environmental Research, 175, 235–256. 10.1016/j.envres.2019.05.013 - DOI - PubMed
-
- Bennett, M. R. , & Devarajan, P. (2011). Characteristics of an ideal biomarker of kidney diseases. In Biomarkers of kidney disease (pp. 1–24). Elsevier. 10.1016/B978-0-12-375672-5.10001-5 - DOI
LinkOut - more resources
Full Text Sources
