Swine IFN cocktail can reduce mortality and lessen the tissue injury caused by African swine-fever-virus-infected piglets
- PMID: 39691698
- PMCID: PMC11649406
- DOI: 10.3389/fcimb.2024.1388035
Swine IFN cocktail can reduce mortality and lessen the tissue injury caused by African swine-fever-virus-infected piglets
Abstract
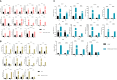
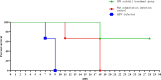
African swine fever (ASF), a highly virulent viral infection, poses a significant threat to the global pig industry. Currently, there are no commercially available vaccines against ASF. While the crucial role of interferon (IFN) in combating viral infections is well-established, its impact on the clinical signs and mortality rates of ASF remains unclear. In this study, swine IFN-α2, IFN-γ, and IFN-λ3 were fused with the Fc segment of immunoglobulin G (IgG) and expressed in mammalian cells (293T), and the antiviral efficacy were detected by VSV-3D4/2 and VSV-PK15 systems. Then, the interferon stimulating genes (ISGs) induced by IFNs-hFc in 3D4/2 cells were determined by qRT-PCR. Also, the preventive potential of the interferon (IFN) cocktail (a mixture of IFNα2-hFc, IFNγ-hFc, and IFNλ3-hFc) were evaluated in vivo by 25-day-old piglets. The results showed that the specific activities of IFNα2-hFc, IFNγ-hFc, and IFNλ3-hFc were 2.46 × 107 IU/mL, 4.54 × 109 IU/mL and 7.54 × 1010 IU/mL, respectively. The IFN-hFc significantly induced the expression of various IFN-stimulated genes (ISGs) in 3D4/2 cells after IFNs-Fc treatment, including IFIT5, Mx1, OASL, ISG12, STAT1, IRF1, PKR, CXCL10, and GBP1. Furthermore, the IFN cocktail treatment reduced the viral load, delayed death, and reduced tissue injury in the piglets infected with ASF virus (ASFV). in conclusion, these results suggest that the IFNs-hFc showed high anti-viral activity, and the IFN cocktail may be potential for the prevention and treatment of ASF.
Keywords: African swine fever; cocktail; interferon; mortality; viral load.
Copyright © 2024 Jiang, Jiang, Zhai, Huang, Pang, Tao, Wang, He, Chu, Zhu, Wu and Jia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

