Human airway epithelium controls Pseudomonas aeruginosa infection via inducible nitric oxide synthase
- PMID: 39691712
- PMCID: PMC11649544
- DOI: 10.3389/fimmu.2024.1508727
Human airway epithelium controls Pseudomonas aeruginosa infection via inducible nitric oxide synthase
Abstract
Introduction: Airway epithelial cells play a central role in the innate immune response to invading bacteria, yet adequate human infection models are lacking.
Methods: We utilized mucociliary-differentiated human airway organoids with direct access to the apical side of epithelial cells to model the initial phase of Pseudomonas aeruginosa respiratory tract infection.
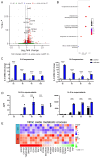
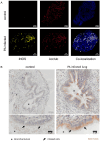
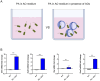
Results: Immunofluorescence of infected organoids revealed that Pseudomonas aeruginosa invades the epithelial barrier and subsequently proliferates within the epithelial space. RNA sequencing analysis demonstrated that Pseudomonas infection stimulated innate antimicrobial immune responses, but specifically enhanced the expression of genes of the nitric oxide metabolic pathway. We demonstrated that activation of inducible nitric oxide synthase (iNOS) in airway organoids exposed bacteria to nitrosative stress, effectively inhibiting intra-epithelial pathogen proliferation. Pharmacological inhibition of iNOS resulted in expansion of bacterial proliferation whereas a NO producing drug reduced bacterial numbers. iNOS expression was mainly localized to ciliated epithelial cells of infected airway organoids, which was confirmed in primary human lung tissue during Pseudomonas pneumonia.
Discussion: Our findings highlight the critical role of epithelial-derived iNOS in host defence against Pseudomonas aeruginosa infection. Furthermore, we describe a human tissue model that accurately mimics the airway epithelium, providing a valuable framework for systemically studying host-pathogen interactions in respiratory infections.
Keywords: Pseudomonas aeruginosa; airway epithelia; airway organoids; iNOS; innate immunity.
Copyright © 2024 Grubwieser, Böck, Soto, Hilbe, Moser, Seifert, Dichtl, Govrins, Posch, Sonnweber, Nairz, Theurl, Trajanoski and Weiss.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- GBD Collaborators LRI . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. (2018) 18:1191–210. doi: 10.1016/s1473-3099(18)30310-4 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

