Exploring lymphocyte subsets in COVID-19 patients: insights from a tertiary academic medical center with a high proportion of patients on immunosuppression
- PMID: 39691720
- PMCID: PMC11649503
- DOI: 10.3389/fimmu.2024.1436637
Exploring lymphocyte subsets in COVID-19 patients: insights from a tertiary academic medical center with a high proportion of patients on immunosuppression
Abstract
Introduction: Severe COVID-19 is associated with reduced absolute lymphocyte counts, suggesting that lymphocyte subsets may serve as predictors of clinical outcomes in affected patients. Early identification of patients at risk for severe disease is crucial for optimizing care, accurately informing patients and their families, guiding therapeutic interventions, and improving patient flow in the ED. Given that immunosuppressive drugs significantly impact lymphocyte profiles, we aimed to determine the association between prior use of immunosuppressive drugs, lymphocyte subsets, and COVID-19 severity in our population with a high prevalence of immunosuppression.
Methods: In 2021, suspected COVID-19 patients were included in the ED. Lymphocyte subsets were determined in peripheral blood within 24 hours after presentation and comparative analyses was performed between SARS-CoV-2 negative and positive patients, mild versus severe disease and patients with and without prior immunosuppressive drug use. Mild cases were patients discharged home or admitted to a general ward, severe cases were patients with COVID-19-related mortality or necessitating ICU admission. Logistic regression analysis was performed to assess the association between lymphocyte subsets and COVID-19 severity, and between prior immunosuppressive drug use and COVID-19 severity.
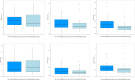
Results: Twenty-five SARS-CoV-2 negative and 77 SARS-CoV-2 positive patients were included, whereof 57 (74%) had mild and 20 (26%) severe COVID-19. No significant differences were observed in the absolute counts of CD3+, CD4+, and CD8+ T-lymphocytes, B-lymphocytes, and NK-cells between SARS-CoV-2 negative and positive patients or between mild and severe cases. The 36 patients with prior use of immunosuppressive drugs had significantly lower CD4+ T-lymphocytes (p<0.01). Prior use of immunosuppressive drugs was not associated with COVID-19 severity (adjusted OR 1.074, 0.355-3.194).
Conclusion: Lymphocyte subsets were not significantly different between SARS-CoV-2 negative and positive patients and between mild versus severe cases. Neither lymphocyte subsets nor prior immunosuppressive drug use were associated with COVID-19 severity.
Keywords: COVID-19; immunosuppression; infectious diseases; lymphocyte subsets; prediction; severity.
Copyright © 2024 Daenen, van Hooijdonk, Tong-Minh, Dik, van Hagen, Huijben, Gommers, van Gorp, Endeman and Dalm.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
CD4 and CD8 Lymphocyte Counts as Surrogate Early Markers for Progression in SARS-CoV-2 Pneumonia: A Prospective Study.Viruses. 2020 Nov 9;12(11):1277. doi: 10.3390/v12111277. Viruses. 2020. PMID: 33182268 Free PMC article.
-
Dynamic Changes in Lymphocyte Populations and Their Relationship with Disease Severity and Outcome in COVID-19.Int J Mol Sci. 2024 Nov 6;25(22):11921. doi: 10.3390/ijms252211921. Int J Mol Sci. 2024. PMID: 39595989 Free PMC article.
-
Dynamic changes in lymphocyte subsets and parallel cytokine levels in patients with severe and critical COVID-19.BMC Infect Dis. 2021 Jan 18;21(1):79. doi: 10.1186/s12879-021-05792-7. BMC Infect Dis. 2021. PMID: 33461503 Free PMC article.
-
Lymphocyte Subset Counts in COVID-19 Patients: A Meta-Analysis.Cytometry A. 2020 Aug;97(8):772-776. doi: 10.1002/cyto.a.24172. Epub 2020 Jul 18. Cytometry A. 2020. PMID: 32542842 Free PMC article. Review.
-
Inflammatory arthritis in patients with COVID-19.Transl Res. 2021 Jun;232:49-59. doi: 10.1016/j.trsl.2021.02.010. Epub 2021 Feb 21. Transl Res. 2021. PMID: 33626415 Free PMC article. Review.
References
-
- WHO Coronavirus (COVID-19) Dashboard . Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed 6 May 2024).
-
- Bahremand T, Yao JA, Mill C, Piszczek J, Grant JM, Smolina K. COVID-19 hospitalisations in immunocompromised individuals in the Omicron era: a population-based observational study using surveillance data in British Columbia, Canada. Lancet Reg Health Am. (2023) 20:100461. doi: 10.1016/j.lana.2023.100461 - DOI - PMC - PubMed
-
- Grivas P, Khaki AR, Wise-Draper TM, French B, Hennessy C, Hsu CY, et al. . Painter CA et al: Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. (2021) 32:787–800. doi: 10.1016/j.annonc.2021.02.024 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

