Preclinical safety assessment in rats after dermal exposure to acetylcarvacrol, a potential acaricide against the brown dog tick
- PMID: 39691818
- PMCID: PMC11650272
- DOI: 10.1016/j.toxrep.2024.101834
Preclinical safety assessment in rats after dermal exposure to acetylcarvacrol, a potential acaricide against the brown dog tick
Abstract
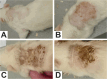
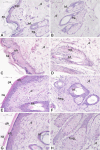
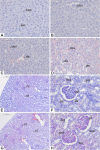
Carvacrol, a phenolic monoterpene found in essential oils of plants of the Lamiaceae family, emerges as an alternative acaricide of plant origin. Its acetylation was proposed to obtain a derivative compound with a better pharmacological profile and lower toxicity to non-target organisms. The present study aimed to assess the preclinical safety of acetylcarvacrol after dermal application in Wistar rats, through the examination of hematological and biochemical parameters, as well as histopathological analysis of the skin, liver and kidney. For this, twenty rats were distributed into four groups with five animals each. Three groups received treatment with different concentrations of the substance (26, 52, and 104 µL/mL) based on the lethal concentration for Rhipicephalus sanguineus ticks, and one group (Control) received only the vehicle. Acetylcarvacrol was applied daily to a trichotomized skin area for 21 days. No changes in hematological parameters were observed. Regarding biochemical analysis, a slight increase in urea and alanine transaminase levels was noted. No significant changes were observed in the kidney and liver, although the rats had developed cumulative irritant contact dermatitis at the application site, as corroborated by the histopathological analysis of the skin. In general, the results showed that the dermal application of acetylcarvacrol in the experimental conditions described here is safe. However, it can cause signs of mild systemic toxicity and skin irritation at high concentrations, suggesting that this product should be used in lower therapeutic doses and that the development of less aggressive formulations, including the combination with other acaricides, is desirable.
Keywords: Acaricide; Dermal toxicity; Semi-synthetic; Tick control.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Sublethal concentrations of acetylcarvacrol affect reproduction and integument morphology in the brown dog tick Rhipicephalus sanguineus sensu lato (Acari: Ixodidae).Exp Appl Acarol. 2020 Oct;82(2):265-279. doi: 10.1007/s10493-020-00538-7. Epub 2020 Aug 28. Exp Appl Acarol. 2020. PMID: 32857314
-
Repellent activity of acetylcarvacrol and its effects on salivary gland morphology in unfed Rhipicephalus sanguineus sensu lato ticks (Acari: Ixodidae).Ticks Tick Borne Dis. 2021 Sep;12(5):101760. doi: 10.1016/j.ttbdis.2021.101760. Epub 2021 Jun 4. Ticks Tick Borne Dis. 2021. PMID: 34130147
-
Low concentrations of acetylcarvacrol induce drastic morphological damages in ovaries of surviving Rhipicephalus sanguineus sensu lato ticks (Acari: Ixodidae).Micron. 2020 Feb;129:102780. doi: 10.1016/j.micron.2019.102780. Epub 2019 Nov 8. Micron. 2020. PMID: 31775098
-
Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin.Int J Toxicol. 2007;26 Suppl 1:3-106. doi: 10.1080/10915810601163939. Int J Toxicol. 2007. PMID: 17365137 Review.
-
Final report on the safety assessment of Triethylene Glycol and PEG-4.Int J Toxicol. 2006;25 Suppl 2:121-38. doi: 10.1080/10915810600964642. Int J Toxicol. 2006. PMID: 17090481 Review.
References
-
- Adenubi O.T., Ahmed A.S., Fasina F.O., McGaw L.J., Eloff J.N., Naidoo V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: a systematic review and meta-analysis. Ind. Crop. Prod. 2018;123:779–806. doi: 10.1016/j.indcrop.2018.06.075. - DOI
-
- Andre W.P.P., Paiva Junior J.R., Cavalcante G.S., Ribeiro W.L.C., Araújo Filho J.V., Santos J.M.L., Alves A.P.N.N., Monteiro J.P., Morais S.M., Silva I.N.G., Oliveira L.M.B., Abreu F.O.M., Bevilaqua C.M.L. Anthelmintic activity of nanoencapsulated carvacryl acetate against gastrointestinal nematodes of sheep and its toxicity in rodents. Braz. J. Vet. Parasitol.. 2020;29(1):1–16. doi: 10.1590/S1984-29612019098. - DOI - PubMed
LinkOut - more resources
Full Text Sources

