Image analysis techniques for in vivo quantification of cerebrospinal fluid flow
- PMID: 39691852
- PMCID: PMC11651631
- DOI: 10.1007/s00348-023-03719-3
Image analysis techniques for in vivo quantification of cerebrospinal fluid flow
Abstract
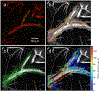
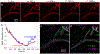
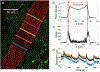
Over the past decade, there has been a tremendously increased interest in understanding the neurophysiology of cerebrospinal fluid (CSF) flow, which plays a crucial role in clearing metabolic waste from the brain. This growing interest was largely initiated by two significant discoveries: the glymphatic system (a pathway for solute exchange between interstitial fluid deep within the brain and the CSF surrounding the brain) and meningeal lymphatic vessels (lymphatic vessels in the layer of tissue surrounding the brain that drains CSF). These two CSF systems work in unison, and their disruption has been implicated in several neurological disorders including Alzheimer's disease, stroke, and traumatic brain injury. Here, we present experimental techniques for in vivo quantification of CSF flow via direct imaging of fluorescent microspheres injected into the CSF. We discuss detailed image processing methods, including registration and masking of stagnant particles, to improve the quality of measurements. We provide guidance for quantifying CSF flow through particle tracking and offer tips for optimizing the process. Additionally, we describe techniques for measuring changes in arterial diameter, which is an hypothesized CSF pumping mechanism. Finally, we outline how these same techniques can be applied to cervical lymphatic vessels, which collect fluid downstream from meningeal lymphatic vessels. We anticipate that these fluid mechanical techniques will prove valuable for future quantitative studies aimed at understanding mechanisms of CSF transport and disruption, as well as for other complex biophysical systems.
Conflict of interest statement
Conflict of Interest The authors have no competing interests to declare that are relevant to the content of this article.
Figures



Update of
-
Image Analysis Techniques for In Vivo Quantification of Cerebrospinal Fluid Flow.bioRxiv [Preprint]. 2023 Jul 24:2023.07.20.549937. doi: 10.1101/2023.07.20.549937. bioRxiv. 2023. Update in: Exp Fluids. 2023 Nov;64(11):181. doi: 10.1007/s00348-023-03719-3. PMID: 37546970 Free PMC article. Updated. Preprint.
References
-
- Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45(4):545–552 - PubMed
-
- Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG (2018) The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 135(3):387–407 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
