Multiomic Integration Analysis for Monitoring Severe Asthma Treated With Mepolizumab or Omalizumab
- PMID: 39692160
- PMCID: PMC12261874
- DOI: 10.1111/all.16434
Multiomic Integration Analysis for Monitoring Severe Asthma Treated With Mepolizumab or Omalizumab
Abstract
Rationale: Biologics are becoming increasingly important in the management of severe asthma. However, little is known about the systemic immunometabolic consequences of Th2 response blockage.
Objectives: To provide a better immunometabolic understanding of the effects of mepolizumab and omalizumab treatments by identifying potential biomarkers for monitoring.
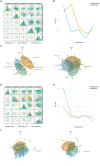
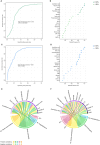
Methods: In this exploratory longitudinal study severe asthmatic patients were followed for 18 months after initiating mepolizumab (n = 36) or Omalizumab (n = 20) treatment. Serum samples were collected before, 6, and 18 months after treatment. Targeted omic approaches were performed to analyze inflammatory metabolites (n = 35) and proteins (n = 45). Multiomic integration was performed individually for each treatment applying supervised analysis Data Integration Analysis for Biomarker discovery using Latent cOmponents (DIABLO) framework. Then, potential biomarkers were confirmed using multivariate ROC analyses and correlated with clinical variables along treatment.
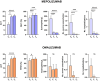
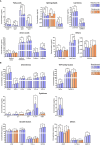
Measurements and main results: Mepolizumab and omalizumab were both effective (improved clinical variables) and showed different and specific metabolic and protein profiles in severe asthmatic patients during treatment. Multiomic integration and multivariate ROC analyses identified specific biomarkers, such as arachidonic acid, palmitoleic acid, oleic acid, propionylcarnitine, bilirubin, CCL11, and TNFSF10, which can explain the differences observed with Mepolizumab treatment over 18 months and significantly correlate with clinical improvement. However, no significant biomolecules and no discriminative multivariate ROC curves were found for Omalizumab treatment.
Conclusions: Our results provide a comprehensive insight into the differential effects of mepolizumab and omalizumab on the immunometabolic kinetics of the inflammatory response in severe asthma. We identified a set of biomolecules with potential for monitoring mepolizumab treatment which could be useful for personalized medicine.
Keywords: asthma; biologicals; biomarkers; metabolomics; proteomics.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
V.M. reports personal fees and/or honoraria from GSK and Astra. C.C. reports personal fees and/or honoraria from Astra, GSK, Sanofi, Gebro, Menarini, and Chiesi and has served Data Safety Monitoring Board and/or Advisory Board at Sanofi, GSK, and Astra. T.C. reports personal fees and/or honoraria from GSK, Sanofi, and AstraZeneca. The other authors declare no conflict of interest.
Figures
References
-
- Agache I. and Akdis C. A., eds. Global Atlas of Asthma, 2nd ed. (Switzerland: European Academy of Allergy and Clinical Immunology, 2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

