Multidisciplinary Approach Improves Eradication Rate and Safety in Refractory Helicobacter pylori Infection
- PMID: 39692308
- PMCID: PMC11845206
- DOI: 10.14309/ctg.0000000000000804
Multidisciplinary Approach Improves Eradication Rate and Safety in Refractory Helicobacter pylori Infection
Abstract
Introduction: Helicobacter pylori (HP) infection is prevalent worldwide and contributes to various gastrointestinal diseases. Eradication therapy is crucial in managing HP infection, but antibiotic resistance has led to refractory cases, complicating treatment outcomes and increasing the risk of adverse events. This study aimed to evaluate the effectiveness of a multidisciplinary approach, termed HP Multidisciplinary Team (MDT) Clinic, in improving eradication rates and safety in patients with refractory HP infection.
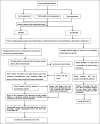
Methods: Between November 2020 and November 2023, 153 patients with refractory HP infection were included, with 51 patients in the non-HP-MDT group and 102 patients in the HP-MDT group. The HP-MDT clinic provided personalized treatment plans, patient education, and follow-up. Genetic testing was conducted in selected cases to assess resistance patterns.
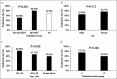
Results: Patients attending the HP-MDT clinic showed significantly higher eradication rates compared with those in the non-HP-MDT group (80.39% vs 50.98%, P < 0.001). Logistic regression analysis confirmed that HP-MDT clinic attendance was independently associated with higher eradication rates (odds ratio: 4.43, 95% CI: 2.02 to 9.71, P < 0.001). Genetic testing revealed high rates of antibiotic resistance, particularly to clarithromycin (10/11, 90.91%) and metronidazole (11/11, 100%). Despite resistance, the HP-MDT approach achieved a high eradication rate of 92.31%. Adverse drug reactions occurred in 12.75% of patients in the HP-MDT group, predominantly mild gastrointestinal symptoms.
Discussion: The HP-MDT clinic, integrating medical, pharmaceutical, and nursing expertise, significantly improved eradication rates and safety in patients with refractory HP infection. Personalized treatment plans, patient education, and genetic testing contributed to successful outcomes with minimal adverse events.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

Similar articles
-
Hybrid Therapy as First-Line Regimen for Helicobacter pylori Eradication in Populations with High Antibiotic Resistance Rates.Helicobacter. 2016 Oct;21(5):382-8. doi: 10.1111/hel.12294. Epub 2016 Jan 25. Helicobacter. 2016. PMID: 26809022
-
Helicobacter pylori eradication therapy outcome according to clarithromycin susceptibility testing in Japan.Helicobacter. 2020 Aug;25(4):e12698. doi: 10.1111/hel.12698. Epub 2020 May 5. Helicobacter. 2020. PMID: 32368846
-
Clarithromycin for first-line treatment of Helicobacter pylori infection after culture in high-resistance regions.Eur J Gastroenterol Hepatol. 2014 Dec;26(12):1380-4. doi: 10.1097/MEG.0000000000000197. Eur J Gastroenterol Hepatol. 2014. PMID: 25229983 Clinical Trial.
-
Host Genetic Determinants Associated With Helicobacter pylori Eradication Treatment Failure: A Systematic Review and Meta-analysis.Gastroenterology. 2021 Nov;161(5):1443-1459. doi: 10.1053/j.gastro.2021.07.043. Epub 2021 Aug 3. Gastroenterology. 2021. PMID: 34358488 Free PMC article.
-
Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance.Front Cell Infect Microbiol. 2017 May 5;7:168. doi: 10.3389/fcimb.2017.00168. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28529929 Free PMC article. Review.
Cited by
-
Adverse event profile of five anti head and neck squamous cell carcinoma drugs: a descriptive analysis from WHO-VigiAccess.Front Pharmacol. 2025 Jun 24;16:1602276. doi: 10.3389/fphar.2025.1602276. eCollection 2025. Front Pharmacol. 2025. PMID: 40630125 Free PMC article.
References
-
- Li Y, Choi H, Leung K, et al. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2023;8(6):553–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

