Pain-Related Quality of Life Outcomes in People With Haemophilia A Receiving Emicizumab: A Post Hoc Analysis of the HAVEN 1, 3 and 4 and STASEY Studies
- PMID: 39692401
- PMCID: PMC11780222
- DOI: 10.1111/hae.15134
Pain-Related Quality of Life Outcomes in People With Haemophilia A Receiving Emicizumab: A Post Hoc Analysis of the HAVEN 1, 3 and 4 and STASEY Studies
Abstract
Introduction: People with haemophilia A (PwHA) experience acute and chronic pain associated with reduced quality of life (QoL).
Aims: This post hoc analysis of pooled data from the HAVEN 1 (NCT02622321), 3 (NCT02847637), 4 (NCT03020160) and STASEY (NCT0319179) studies assessed the impact of emicizumab prophylaxis on pain-related QoL in PwHA.
Methods: PwHA received emicizumab during the four studies. In this analysis, pain was assessed using patient-reported responses to pain-specific questions from the Haem-A-QoL/Haemo-QoL-SF and the pain/discomfort dimension of the EQ-5D-5L. Responses were recorded at baseline and at regular intervals for up to 78 weeks following treatment initiation. Additional analyses evaluated the population with target joints at baseline, and the overall population stratified by age, factor (F)VIII inhibitor status and prior treatment.
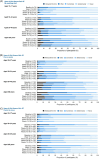
Results: At the data cut-off, 504 PwHA had been treated across the four studies; 464 and 470 completed the Haem-A-QoL/Haemo-QoL-SF and the EQ-5D-5L, respectively. Improvements in pain-related QoL were observed by Week 13 of emicizumab prophylaxis and maintained through Week 78. In the overall population, responses of 'never/rarely' for 'my swellings hurt' and 'pain in joints' increased from 37.0% and 30.0% at baseline to 84.0% and 61.0% by Week 13, suggesting reductions in acute and chronic pain, respectively. Similar improvements were seen in the target joint population, and across all strata. Greater improvements were observed in younger versus older PwHA.
Conclusion: Pain-related QoL improved with emicizumab prophylaxis regardless of target joints, age, FVIII inhibitor status or prior treatment. Haemophilia-specific assessments are needed to accurately capture and characterize pain in PwHA.
Keywords: arthralgia; emicizumab; haemarthrosis; haemophilia; pain; prophylaxis; quality of life.
© 2024 The Author(s). Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
Cedric Hermans has received research funding from Bayer, CSL Behring, Novo Nordisk, Pfizer, Shire/Takeda, and Sobi; consulting fees from Bayer, Shire/Takeda, Novo Nordisk, CSL Behring, Sobi, Pfizer, CAF‐DCF, and LFB; speakers bureau and honoraria from Bayer, BioMarin, CAF‐DCF, CSL Behring, F. Hoffmann‐La Roche Ltd, LFB, Novo Nordisk, Octapharma, Pfizer, Shire/Takeda, Sobi, and UniQure; support for attending meetings and/or travel from BioMarin, F. Hoffmann‐La Roche Ltd, LFB, Novo Nordisk, and Sobi. Mark W. Skinner has received research support for the PROBE study and core outcomes studies as part of independent investigator‐initiated research projects from Band Therapeutics, BioMarin, CSL Behring, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Sanofi, Takeda, and Vega Therapeutics; consulting fees from Bayer, and Sanofi; honoraria from Bayer, Novo Nordisk, Genentech, Inc., Sanofi, and Takeda; support for attending meetings and/or travel from Bayer, Novo Nordisk, Sanofi, Takeda; participation on a Data Safety Monitoring Board or advisory board for Pfizer and Spark Therapeutics, Inc.; serves on advisory or governance boards of Blue Cross Blue Shield MAP, ICER, NHF MASA, NORD and WFH USA. Brittany Gentile has nothing to disclose. Elise Lim, Eunice Tzeng and Miranda Minhas are employees of Genentech, Inc., and shareholders at F. Hoffmann‐La Roche Ltd. Katya Moreno is an employee and shareholder of F. Hoffmann‐La Roche Ltd. Tyler W. Buckner has received research funding from NIH—NHLBI; consulting fees from BioMarin and Tremeau Pharmaceuticals; honoraria from Octapharma; participation on an advisory board for BioMarin, CSL Behring, and Novo Nordisk; board of directors for American Thrombosis and Hemostasis Network.
Figures






References
-
- Srivastava A., Santagostino E., Dougall A., et al., “WFH Guidelines for the Management of Haemophilia, 3rd Edition,” Haemophilia 26, no. S6 (2020): 1–158. - PubMed
-
- Witkop M., Neff A., Buckner T. W., et al., “Self‐Reported Prevalence, Description and Management of Pain in Adults With Haemophilia: Methods, Demographics and Results From the Pain, Functional Impairment, and Quality of Life (P‐FiQ) Study,” Haemophilia 23, no. 4 (2017): 556–565. - PubMed
-
- Chai‐Adisaksopha C., Noone D., Curtis R., et al., “Non‐Severe Haemophilia: Is It Benign? – Insights From the PROBE Study,” Haemophilia 27, no. S1 (2021): 17–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

