Impact of Anti-CD4 Autoantibodies on Immune Reconstitution in People With Advanced HIV
- PMID: 39692471
- PMCID: PMC12272841
- DOI: 10.1093/cid/ciae562
Impact of Anti-CD4 Autoantibodies on Immune Reconstitution in People With Advanced HIV
Abstract
Background: Despite suppressive antiretroviral therapy (ART), 15%-30% of people with human immunodeficiency virus (HIV) experience a limited recovery of CD4 T cells. Although autoantibodies against the CD4 receptor have previously been identified in people with HIV (PWH), little is known about their longitudinal impact on CD4 T-cell reconstitution.
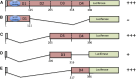
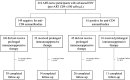
Methods: Anti-CD4 autoantibodies were evaluated by the fluid-phase luciferase immunoprecipitation systems immunoassay in ART-naive people with advanced HIV (CD4 count ≤100 cells/µL), PWH with CD4 count >200 cells/µL, long-term nonprogressors, people with idiopathic CD4 lymphopenia, people with autoimmune lymphoproliferative syndrome, and healthy volunteers without HIV. In the participants with advanced HIV, we assessed the association of anti-CD4 autoantibodies at ART initiation with CD4 recovery over a median follow-up of 192 weeks.
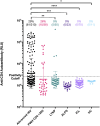
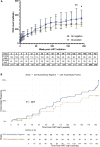
Results: Anti-CD4 autoantibodies were identified in 29% (61/210) of ART-naive participants with advanced HIV but were absent in people without HIV. Female PWH showed a 4-fold higher prevalence (P < .001) of anti-CD4 autoantibodies compared to males. After ART initiation, people with advanced HIV with anti-CD4 autoantibodies exhibited an overall slower rate of CD4 reconstitution (5.8 vs 6.6 cells/µL/month, P = .007) and lower week 192 CD4 count (268 vs 355 cells/µL, P = .037). Incidental, clinically indicated immunosuppressive therapy in these participants was associated with an improved rate of CD4 reconstitution (P = .0019) and higher week 192 CD4 count (551 vs 268 cells/µL, P = .019).
Conclusions: People with advanced HIV harboring anti-CD4 autoantibodies at ART initiation demonstrated a slower rate and extent of CD4 reconstitution after 4 years. Incidental immunosuppressive therapy was associated with increased CD4 counts in these participants.
Keywords: CD4 T cells; HIV; anti-CD4 autoantibodies; immune reconstitution; immunological nonresponse.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2024.
Conflict of interest statement
Potential conflicts of interest . The authors: A.L. is engaged in a formal Cooperative Research and Development Agreement (CRADA) with NeoImmuneTech outside of the submitted work. I.S. reports collaborative research projects with NeoImmunoTech and Karius, Inc. outside of the submitted work. All other authors have no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures


References
-
- Ford N, Ehrenkranz P, Jarvis J. Advanced HIV as a neglected disease. N Engl J Med 2024; 390:487–9. - PubMed
-
- Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis 2007; 44:441–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

