Clinical and genetic characterization of a progressive RBL2-associated neurodevelopmental disorder
- PMID: 39692517
- PMCID: PMC11967543
- DOI: 10.1093/brain/awae363
Clinical and genetic characterization of a progressive RBL2-associated neurodevelopmental disorder
Abstract
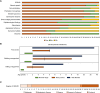
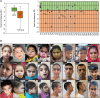
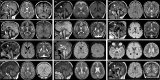
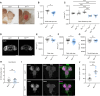
Retinoblastoma (RB) proteins are highly conserved transcriptional regulators that play important roles during development by regulating cell-cycle gene expression. RBL2 dysfunction has been linked to a severe neurodevelopmental disorder. However, to date, clinical features have been described in only six individuals carrying five biallelic predicted loss-of-function (pLOF) variants. To define the phenotypic effects of RBL2 mutations in detail, we identified and clinically characterized a cohort of 35 patients from 20 families carrying pLOF variants in RBL2, including 15 new variants that substantially broaden the molecular spectrum. The clinical presentation of affected individuals is characterized by a range of neurological and developmental abnormalities. Global developmental delay and intellectual disability were observed uniformly, ranging from moderate to profound and involving lack of acquisition of key motor and speech milestones in most patients. Disrupted sleep was also evident in some patients. Frequent features included postnatal microcephaly, infantile hypotonia, aggressive behaviour, stereotypic movements, seizures and non-specific dysmorphic features. Neuroimaging features included cerebral atrophy, white matter volume loss, corpus callosum hypoplasia and cerebellar atrophy. In parallel, we used the fruit fly, Drosophila melanogaster, to investigate how disruption of the conserved RBL2 orthologue Rbf impacts nervous system function and development. We found that Drosophila Rbf LOF mutants recapitulate several features of patients harbouring RBL2 variants, including developmental delay, alterations in head and brain morphology, locomotor defects and perturbed sleep. Surprisingly, in addition to its known role in controlling tissue growth during development, we found that continued Rbf expression is also required in fully differentiated post-mitotic neurons for normal locomotion in Drosophila, and that adult-stage neuronal re-expression of Rbf is sufficient to rescue Rbf mutant locomotor defects. Taken together, our study provides a clinical and experimental basis to understand genotype-phenotype correlations in an RBL2-linked neurodevelopmental disorder and suggests that restoring RBL2 expression through gene therapy approaches might ameliorate some symptoms caused by RBL2 pLOF.
Keywords: Drosophila; RBL2; Rbf; cell cycle; neurodevelopmental disorder.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
C.B. is an employee of Centogene GmbH. G.H.S. is an employee of 3billion. L.M. has received personal fees for
Figures


References
-
- Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–E67. - PubMed
-
- Yao Y, Gu X, Xu X, Ge S, Jia R. Novel insights into RB1 mutation. Cancer Lett. 2022;547:215870. - PubMed
-
- Chai P, Luo Y, Yu J, et al. Clinical characteristics and germline mutation spectrum of RB1 in Chinese patients with retinoblastoma: A dual-center study of 145 patients. Exp Eye Res. 2021;205:108456. - PubMed
-
- Wadayama B, Toguchida J, Shimizu T, et al. Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res. 1994;54:3042–3048. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

