Nose-to-Brain Delivery of Biomimetic Nanoparticles for Glioblastoma Targeted Therapy
- PMID: 39692595
- PMCID: PMC11783514
- DOI: 10.1021/acsami.4c16837
Nose-to-Brain Delivery of Biomimetic Nanoparticles for Glioblastoma Targeted Therapy
Abstract
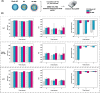
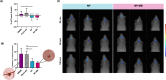
Glioblastoma (GBM) is an extremely aggressive form of brain cancer that remains challenging to treat, especially owing to the lack of effective targeting and drug delivery concerns. Due to its anatomical advantages, the nose-to-brain strategy is an interesting route for drug delivery. Nanoengineering has provided technological tools and innovative strategies to overcome biotechnological limitations, which is promising for improving the effectiveness of conventional therapies. Herein, we designed a biomimetic multifunctional nanostructure produced by polymeric poly(d,l-lactic-co-glycolic) acid (PLGA) core loaded with Temozolomide (TMZ) coated with cell membrane isolated from glioma cancer cells. The developed nanostructures (NP-MB) were fully characterized, and their biological performance was investigated extensively. The results indicate that NP-MB could control TMZ release and promote TMZ permeation in the ex vivo nasal porcine mucosa. The higher cytotoxicity of NP-MB in different glioma cell lines, particularly against U251 cells, reinforces their potential for homotypic targeting. The chicken chorioallantoic membrane assay revealed a tumor size reduction and antiangiogenic activity. In vivo biodistribution studies showed that NP-MB effectively reaches the brain following nasal administration. These findings suggest that NP-MB holds promise as a biomimetic nanoplatform for effective targeting and homotypic recognition in GBM therapy with high potential for clinical translation.
Keywords: PLGA-based nanoparticles; Temozolomide; biomimetic delivery systems; glioblastoma treatment; homotypic recognition; nanotechnology; nose-to-brain delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Enhanced Caspase-Mediated Abrogation of Autophagy by Temozolomide-Loaded and Panitumumab-Conjugated Poly(lactic-co-glycolic acid) Nanoparticles in Epidermal Growth Factor Receptor Overexpressing Glioblastoma Cells.Mol Pharm. 2020 Nov 2;17(11):4386-4400. doi: 10.1021/acs.molpharmaceut.0c00856. Epub 2020 Oct 20. Mol Pharm. 2020. PMID: 33079558
-
Development of transferrin-modified poly(lactic-co-glycolic acid) nanoparticles for glioma therapy.Anticancer Drugs. 2019 Jul;30(6):604-610. doi: 10.1097/CAD.0000000000000754. Anticancer Drugs. 2019. PMID: 30855310
-
Chitosan-PLGA mucoadhesive nanoparticles for gemcitabine repurposing for glioblastoma therapy.Eur J Pharm Biopharm. 2024 Jul;200:114326. doi: 10.1016/j.ejpb.2024.114326. Epub 2024 May 15. Eur J Pharm Biopharm. 2024. PMID: 38759897
-
Self-Assembled systems for Nose-to-Brain delivery of Temozolamide (TMZ) in brain tumor therapy.Int J Pharm. 2025 Apr 30;675:125540. doi: 10.1016/j.ijpharm.2025.125540. Epub 2025 Mar 31. Int J Pharm. 2025. PMID: 40174811 Review.
-
Improving temozolomide biopharmaceutical properties in glioblastoma multiforme (GBM) treatment using GBM-targeting nanocarriers.Eur J Pharm Biopharm. 2021 Nov;168:76-89. doi: 10.1016/j.ejpb.2021.08.011. Epub 2021 Aug 28. Eur J Pharm Biopharm. 2021. PMID: 34461214 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

