Covalent Proximity Inducers
- PMID: 39692621
- PMCID: PMC11719315
- DOI: 10.1021/acs.chemrev.4c00570
Covalent Proximity Inducers
Abstract
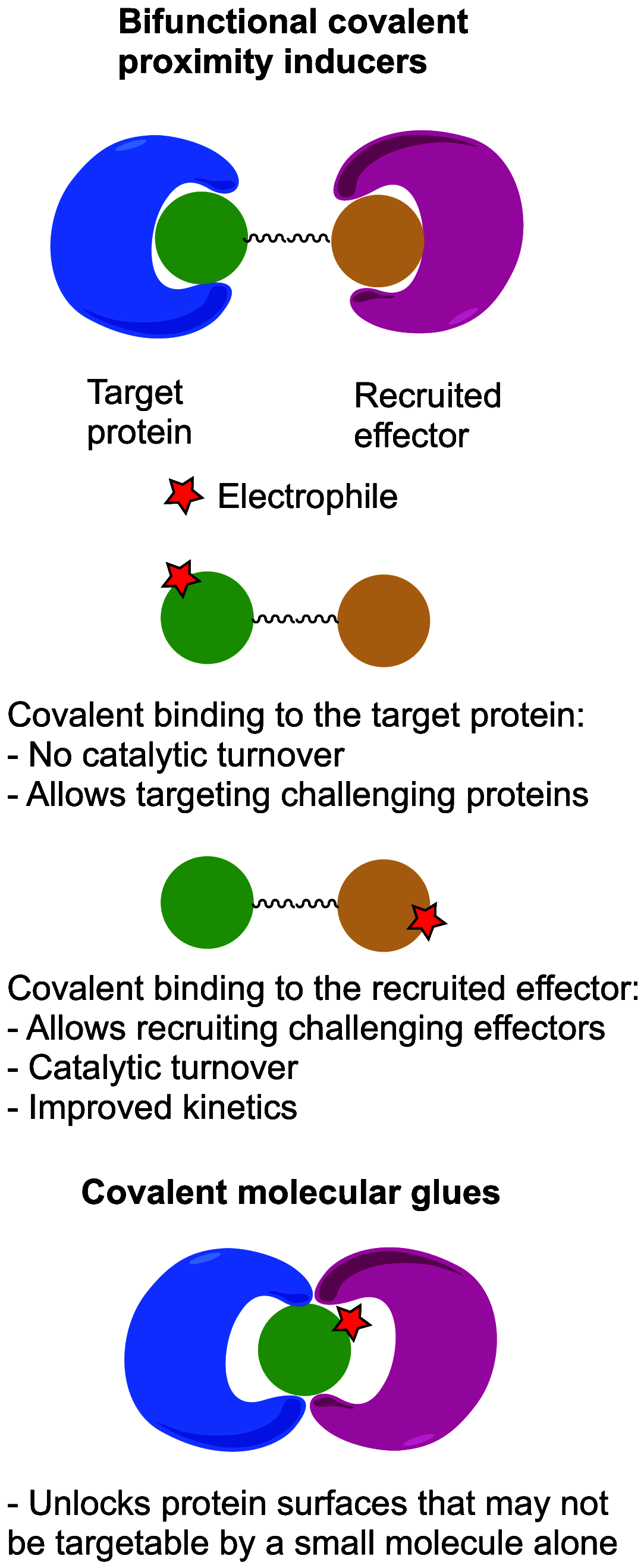
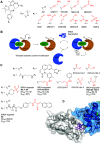
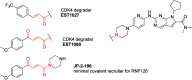
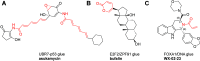
Molecules that are able to induce proximity between two proteins are finding ever increasing applications in chemical biology and drug discovery. The ability to introduce an electrophile and make such proximity inducers covalent can offer improved properties such as selectivity, potency, duration of action, and reduced molecular size. This concept has been heavily explored in the context of targeted degradation in particular for bivalent molecules, but recently, additional applications are reported in other contexts, as well as for monovalent molecular glues. This is a comprehensive review of reported covalent proximity inducers, aiming to identify common trends and current gaps in their discovery and application.
Conflict of interest statement
The author declares the following competing financial interest(s): N.L. serves on the SAB of Larkspur Biosciences, Tesseract Medicines and Eindura Therapeutics.
Figures

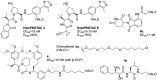
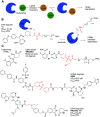
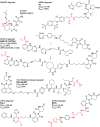
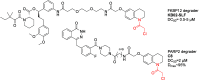
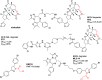

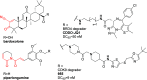
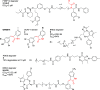

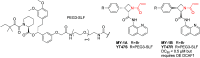
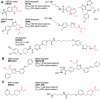
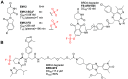

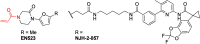

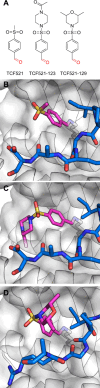


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

