The Na+,K+,2Cl- Cotransporter, Not Aquaporin 1, Sustains Cerebrospinal Fluid Secretion While Controlling Brain K+ Homeostasis
- PMID: 39692709
- PMCID: PMC11809428
- DOI: 10.1002/advs.202409120
The Na+,K+,2Cl- Cotransporter, Not Aquaporin 1, Sustains Cerebrospinal Fluid Secretion While Controlling Brain K+ Homeostasis
Abstract
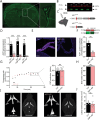
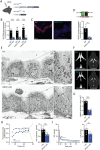
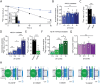
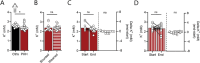
Disturbances in the brain fluid balance can lead to life-threatening elevation in intracranial pressure (ICP), which represents a vast clinical challenge. Targeted and efficient pharmaceutical therapy of elevated ICP is not currently available, as the molecular mechanisms governing cerebrospinal fluid (CSF) secretion are largely unresolved. To resolve the quantitative contribution of key choroid plexus transport proteins, this study employs mice with genetic knockout and/or viral choroid plexus-specific knockdown of aquaporin 1 (AQP1) and the Na+, K+, 2Cl- cotransporter 1 (NKCC1) for in vivo determinations of CSF dynamics, ex vivo choroid plexus for transporter-mediated clearance of a CSF K+ load, and patient CSF for [K+] quantification. CSF secretion and ICP management occur independently of choroid plexus AQP1 expression, whereas both parameters are reduced by 40% upon choroid plexus NKCC1 knockdown. Elevation of [K+]CSF increases the choroid plexus Na+/K+-ATPase activity, and favors inwardly-directed net NKCC1 transport, which, together, promote CSF K+ clearance, while maintaining undisturbed CSF secretion rates. CSF from patients with post-hemorrhagic hydrocephalus does not display elevated [K+]CSF, suggesting that NKCC1 maintains net outward transport direction during post-hemorrhagic hydrocephalus formation. Direct or indirect therapeutic modulation of choroid plexus NKCC1 can thus be a potential promising pharmacological approach against brain pathologies associated with elevated ICP.
Keywords: CSF; K+; NKCC1; aquaporin; choroid plexus; hydrocephalus.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
