D609 Suppresses Antituberculosis Response by Regulating Dendritic Cells Antigen Presentation
- PMID: 39692711
- PMCID: PMC11653942
- DOI: 10.1002/iid3.70103
D609 Suppresses Antituberculosis Response by Regulating Dendritic Cells Antigen Presentation
Abstract
Objective: To elucidate the role of phosphatidylcholine-specific phospholipase C (PC-PLC) in the antituberculosis (anti-TB) immune response mediated by dendritic cells (DCs).
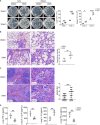
Methods: In vivo, C57BL/6J mice infected with the Mycobacterium tuberculosis strain H37Rv. Before infection, the mice were pretreated with the PC-PLC inhibitor D609. Bacillary loads in lung and spleen tissues were quantified through colony-forming unit (CFU) assays. Hematoxylin and eosin (H&E) staining was performed to assess inflammatory infiltration and tissue damage. Levels of inflammatory mediators in peripheral venous blood were quantified using enzyme-linked immunosorbent assays (ELISAs). Flow cytometry was employed to determine the proportions of conventional DCs (cDCs) and their subsets, cDC1 and cDC2, within lung, spleen, and lymph node tissues. In vitro, mouse bone marrow-derived dendritic cells (BMDCs) pretreated with D609. The expression levels of chemokines and pro-inflammatory cytokines were assessed via quantitative polymerase chain reaction (qPCR) and ELISA. BMDCs were loaded with H37Rv expressing red fluorescent protein (RFP-H37Rv) or DQ-OVA, and flow cytometry was utilized to analyze the impact of D609 on antigen phagocytosis and processing. Furthermore, flow cytometry was employed to evaluate the effect of D609 pretreatment on the expression levels of costimulatory molecules on BMDCs. The capacity of D609-treated BMDCs to activate and proliferate T cells, as well as to induce interferon-gamma (IFN-γ) secretion, was assessed through a DC-T cell coculture system.
Results: In vivo analysis revealed that mice pretreated with D609 exhibited a marked increase in tissue bacterial load, enhanced inflammatory infiltration, and a reduction in pro-inflammatory mediator expression in peripheral venous blood. There was a notable decrease in the number of cDCs in lung and lymph node tissues, with a pronounced reduction in cDC1 in the lungs and cDC2 in the lymph nodes. In vitro studies demonstrated that D609 pretreated BMDCs displayed a significant decline in inflammatory mediator production, antigen phagocytosis, and antigen processing capabilities, potentially due to altered expression of costimulatory molecules. Coculture experiments indicated that D609 pretreated BMDCs showed a substantial reduction in their ability to stimulate T cell activation, proliferation, and IFN-γ secretion.
Conclusion: Our findings suggest that PC-PLC plays a critical role in the functionality of DCs, including the production of chemokines and pro-inflammatory cytokines, migration to lymph nodes, and antigen presentation to T cells, which collectively contribute to T cell activation and effective clearance of Mycobacterium tuberculosis. Further investigation into the regulatory mechanisms of PC-PLC in DCs may uncover novel therapeutic targets for the development of advanced anti-TB treatments.
Keywords: D609; Mycobacterium tuberculosis; antigen presentation; dendritic cells.
© 2024 The Author(s). Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Mycobacterium tuberculosis GrpE, A Heat-Shock Stress Responsive Chaperone, Promotes Th1-Biased T Cell Immune Response via TLR4-Mediated Activation of Dendritic Cells.Front Cell Infect Microbiol. 2018 Mar 27;8:95. doi: 10.3389/fcimb.2018.00095. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29637049 Free PMC article.
-
Aerosol immunization by alginate coated mycobacterium (BCG/MIP) particles provide enhanced immune response and protective efficacy than aerosol of plain mycobacterium against M.tb. H37Rv infection in mice.BMC Infect Dis. 2019 Jul 1;19(1):568. doi: 10.1186/s12879-019-4157-2. BMC Infect Dis. 2019. PMID: 31262260 Free PMC article.
-
Specificity and efficacy of dendritic cell-based vaccination against tuberculosis with complex mycobacterial antigens in a mouse model.Tuberculosis (Edinb). 2007 Mar;87(2):134-44. doi: 10.1016/j.tube.2006.06.002. Epub 2006 Oct 2. Tuberculosis (Edinb). 2007. PMID: 17011827
-
The role of ion channels in the regulation of dendritic cell function.Cell Calcium. 2025 Jun;128:103031. doi: 10.1016/j.ceca.2025.103031. Epub 2025 Apr 16. Cell Calcium. 2025. PMID: 40253771 Review.
-
Cellular respiration in dendritic cells: Exploring oxygen-dependent pathways for potential therapeutic interventions.Free Radic Biol Med. 2025 Feb 1;227:536-556. doi: 10.1016/j.freeradbiomed.2024.12.014. Epub 2024 Dec 4. Free Radic Biol Med. 2025. PMID: 39643130 Review.
References
-
- Bagcchi S., “WHO's Global Tuberculosis Report 2022,” Lancet Microbe 4, no. 1 (2023): e20. - PubMed
-
- Shao J., Sun C., Su L., Zhao J., Zhang S., and Miao J., “Phosphatidylcholine‐Specific Phospholipase C/Heat Shock Protein 70 (Hsp70)/transcription Factor B‐Cell Translocation Gene 2 Signaling in Rat Bone Marrow Stromal Cell Differentiation to Cholinergic Neuron‐Like Cells,” International Journal of Biochemistry & Cell Biology 44, no. 12 (2012): 2253–2260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

