Circadian rhythms and cancer: implications for timing in therapy
- PMID: 39692981
- PMCID: PMC11655929
- DOI: 10.1007/s12672-024-01643-4
Circadian rhythms and cancer: implications for timing in therapy
Abstract
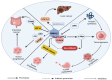
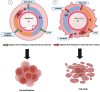
Circadian rhythms, intrinsic cycles spanning approximately 24 h, regulate numerous physiological processes, including sleep-wake cycles, hormone release, and metabolism. These rhythms are orchestrated by the circadian clock, primarily located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Disruptions in circadian rhythms, whether due to genetic mutations, environmental factors, or lifestyle choices, can significantly impact health, contributing to disorders such as sleep disturbances, metabolic syndrome, and cardiovascular diseases. Additionally, there is a profound link between the disruption of circadian rhythms and development of various cancer, the influence on disease incidence and progression. This incurred regulation by circadian clock on pathways has its implication in tumorigenesis, such as cell cycle control, DNA damage response, apoptosis, and metabolism. Furthermore, the circadian timing system modulates the efficacy and toxicity of cancer treatments. In cancer treatment, the use of chronotherapy to optimize the timing of medical treatments, involves administering chemotherapy, radiation, or other therapeutic interventions at specific intervals to enhance efficacy and minimize side effects. This approach capitalizes on the circadian variations in cellular processes, including DNA repair, cell cycle progression, and drug metabolism. Preclinical and clinical studies have demonstrated that chronotherapy can significantly improve the therapeutic index of chemotherapeutic agents like cisplatin and 5-fluorouracil by enhancing anticancer activity and reducing toxicity. Further research is needed to elucidate the mechanisms underlying circadian regulation of cancer and to develop robust chronotherapeutic protocols tailored to individual patients' circadian profiles, potentially transforming cancer care into more effective and personalized treatment strategies.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
