Brexpiprazole and Sertraline Combination Treatment in Posttraumatic Stress Disorder: A Phase 3 Randomized Clinical Trial
- PMID: 39693081
- PMCID: PMC11883513
- DOI: 10.1001/jamapsychiatry.2024.3996
Brexpiprazole and Sertraline Combination Treatment in Posttraumatic Stress Disorder: A Phase 3 Randomized Clinical Trial
Abstract
Importance: New pharmacotherapy options are needed for posttraumatic stress disorder (PTSD).
Objective: To investigate the efficacy, safety, and tolerability of brexpiprazole and sertraline combination treatment (brexpiprazole + sertraline) compared with sertraline + placebo for PTSD.
Design, setting, and participants: This was a parallel-design, double-blind, randomized clinical trial conducted from October 2019 to August 2023. The study had a 1-week, placebo run-in period followed by an 11-week, double-blind, randomized, active-controlled, parallel-arm period (with 21-day follow-up) and took place at 86 clinical trial sites in the US. Adult outpatients with PTSD were enrolled (volunteer sample).
Interventions: Oral brexpiprazole 2 to 3 mg per day (flexible dose) + sertraline 150 mg per day or sertraline 150 mg per day + placebo (1:1 ratio) for 11 weeks.
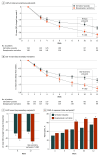
Main outcomes and measures: The primary end point was change in Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) total score (which measures the severity of 20 PTSD symptoms) from randomization (week 1) to week 10 for brexpiprazole + sertraline vs sertraline + placebo. Safety assessments included adverse events.
Results: A total of 1327 individuals were assessed for eligibility. After 878 screen failures, 416 participants (mean [SD] age, 37.4 [11.9] years; 310 female [74.5%]) were randomized. Completion rates were 137 of 214 participants (64.0%) for brexpiprazole + sertraline and 113 of 202 participants (55.9%) for sertraline + placebo. At week 10, brexpiprazole + sertraline demonstrated statistically significant greater improvement in CAPS-5 total score (mean [SD] at randomization, 38.4 [7.2]; LS mean [SE] change, -19.2 [1.2]; n = 148) than sertraline + placebo (randomization, 38.7 [7.8]; change, -13.6 [1.2]; n = 134), with LS mean difference, -5.59 (95% CI, -8.79 to -2.38; P < .001). All key secondary and other efficacy end points were also met. Treatment-emergent adverse events with incidence of 5% or greater for brexpiprazole + sertraline (and corresponding incidences for sertraline + placebo) were nausea (25 of 205 [12.2%] and 23 of 196 [11.7%]), fatigue (14 of 205 [6.8%] and 8 of 196 [4.1%]), weight increase (12 of 205 [5.9%] and 3 of 196 [1.5%]), and somnolence (11 of 205 [5.4%] and 5 of 196 [2.6%]). Discontinuation rates due to adverse events were 8 of 205 participants (3.9%) for brexpiprazole + sertraline and 20 of 196 participants (10.2%) for sertraline + placebo.
Conclusions and relevance: Results of this randomized clinical trial show that brexpiprazole + sertraline combination treatment statistically significantly improved PTSD symptoms vs sertraline + placebo, indicating its potential as a new efficacious treatment for PTSD. Brexpiprazole + sertraline was tolerated by most participants, with a safety profile consistent with that of brexpiprazole in approved indications.
Trial registration: ClinicalTrials.gov Identifier: NCT04124614.
Conflict of interest statement
Figures

References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition, Text Revision. American Psychiatric Association; 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

