Consensus Recommendations for Studies of Outflow Facility and Intraocular Pressure Regulation Using Ex Vivo Perfusion Approaches
- PMID: 39693082
- PMCID: PMC11708870
- DOI: 10.1167/iovs.65.14.32
Consensus Recommendations for Studies of Outflow Facility and Intraocular Pressure Regulation Using Ex Vivo Perfusion Approaches
Abstract
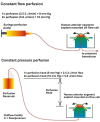
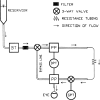
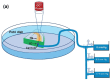
Intraocular pressure (IOP) elevation is the primary risk factor and currently the main treatable factor for progression of glaucomatous optic neuropathy. In addition to direct clinical and living animal in vivo studies, ex vivo perfusion of anterior segments and whole eyes is a key technique for studying conventional outflow function as it is responsible for IOP regulation. We present well-tested experimental details, protocols, considerations, advantages, and limitations of several ex vivo model systems for studying IOP regulation. These include: (1) perfused whole globes, (2) stationary anterior segment organ culture, (3) perfused human anterior segment organ culture, (4) perfused animal anterior segment organ culture, (5) perfused human corneal rims, and (6) perfused human anterior segment wedges. These methods, with due consideration paid to their strengths and limitations, comprise a set of very strong tools for extending our understanding of IOP regulation.
Conflict of interest statement
Disclosure:
Figures











References
MeSH terms
Grants and funding
- R01 EY033015/EY/NEI NIH HHS/United States
- R01 EY023242/EY/NEI NIH HHS/United States
- R01 EY019643/EY/NEI NIH HHS/United States
- R01 EY031817/EY/NEI NIH HHS/United States
- R01 EY032590/EY/NEI NIH HHS/United States
- R01 EY030501/EY/NEI NIH HHS/United States
- P30 EY014800/EY/NEI NIH HHS/United States
- R01 EY034096/EY/NEI NIH HHS/United States
- R01 EY022634/EY/NEI NIH HHS/United States
- R21 EY033961/EY/NEI NIH HHS/United States
- R21 EY028671/EY/NEI NIH HHS/United States
- P30 EY005722/EY/NEI NIH HHS/United States
- P30 EY010572/EY/NEI NIH HHS/United States
- R01 EY026048/EY/NEI NIH HHS/United States
- R01 EY026962/EY/NEI NIH HHS/United States
- R21 EY033073/EY/NEI NIH HHS/United States
- R01 EY030238/EY/NEI NIH HHS/United States
- R01 EY025643/EY/NEI NIH HHS/United States
- R01 EY026885/EY/NEI NIH HHS/United States
- R01 EY003279/EY/NEI NIH HHS/United States
- R01 EY031700/EY/NEI NIH HHS/United States
- R01 EY034238/EY/NEI NIH HHS/United States
- R01 EY022359/EY/NEI NIH HHS/United States
- R01 EY021205/EY/NEI NIH HHS/United States
- R01 EY027920/EY/NEI NIH HHS/United States
- R01 EY036011/EY/NEI NIH HHS/United States
- R21 EY036189/EY/NEI NIH HHS/United States
- R01 EY033600/EY/NEI NIH HHS/United States
- R01 EY025721/EY/NEI NIH HHS/United States
- R21 EY031071/EY/NEI NIH HHS/United States
- R01 EY032960/EY/NEI NIH HHS/United States
- R01 EY026177/EY/NEI NIH HHS/United States
- R21 EY033929/EY/NEI NIH HHS/United States
- P30 EY031631/EY/NEI NIH HHS/United States
- R01 EY008247/EY/NEI NIH HHS/United States
- R01 EY021800/EY/NEI NIH HHS/United States
- R01 EY026529/EY/NEI NIH HHS/United States

