The transcription factor OsNAC25 regulates potassium homeostasis in rice
- PMID: 39693105
- PMCID: PMC11869173
- DOI: 10.1111/pbi.14550
The transcription factor OsNAC25 regulates potassium homeostasis in rice
Abstract
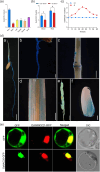
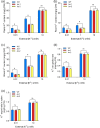
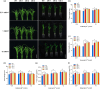
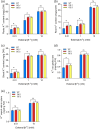
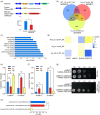
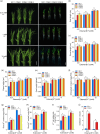
Over-application of potassium (K) fertilizer in fields has a negative impact on the environment. Developing rice varieties with high KUE will reduce fertilizer for sustainable agriculture. However, the genetic basis of KUE in a more diverse and inclusive population remains largely unexplored. Here, we show that the transcription factor OsNAC25 enhances K+ uptake and confers high KUE under low K+ supply. Disruption of OsNAC25 by CRISPR/Cas9-mediated mutagenesis led to a considerable loss of K+ uptake capacity in rice roots, coupled with reduced K+ accumulation in rice and severe plant growth defects under low- K+ conditions. However, the overexpression of OsNAC25 enhanced K+ accumulation by regulating proper K+ uptake capacity in rice roots. Further analysis displayed that OsNAC25 can bind to the promoter of OsSLAH3 to repress its transcription in response to low- K+ stress. Nucleotide diversity analyses suggested that OsNAC25 may be selected during japonica populations' adaptation of low K+ tolerance. Natural variation of OsNAC25 might cause differential expression in different haplotype varieties, thus conferring low K+ tolerance in the Hap 1 and Hap 4 -carrying varieties, and the japonica allele OsNAC25 could enhance low K+ tolerance in indica variety, conferring great potential to improve indica low K+ tolerance and grain development. Taken together, we have identified a new NAC regulator involved in rice low K+ tolerance and grain development, and provide a potential target gene for improving low K+ tolerance and grain development in rice.
Keywords: K+ uptake; OsNAC25; Potassium; low K+ stress; natural variation; rice.
© 2024 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Adams, E. and Shin, R. (2014) Transport, signaling, and homeostasis of potassium and sodium in plants. J. Integr. Plant Biol. 56, 231–249. - PubMed
-
- Cheong, Y.H. , Pandey, G.K. , Gran, J.J. , Batistic, O. , Li, L.G. , Kim, B.G. , Lee, S.C. et al. (2007) Two calcineurin B‐like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 52, 223–239. - PubMed
-
- Clarkson, D.T. and Hanson, J.B. (1980) The mineral nutrition of higher plants. Annu. Rev. Plant Physiol. 31, 239–298.
MeSH terms
Substances
Grants and funding
- 2021JJ30013/the National Science Foundation of Hunan province
- 2022JJ30382/the National Science Foundation of Hunan province
- 20C1124/the Research Foundation of Education Bureau of Hunan Province of China
- 20A295/the Research Foundation of Education Bureau of Hunan Province of China
- 22A0018/the Research Foundation of Education Bureau of Hunan Province of China
LinkOut - more resources
Full Text Sources
Medical

