Central nervous system vascularization in human embryos and neural organoids
- PMID: 39693224
- PMCID: PMC11975460
- DOI: 10.1016/j.celrep.2024.115068
Central nervous system vascularization in human embryos and neural organoids
Abstract
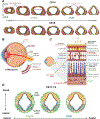
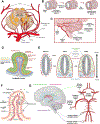
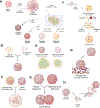
In recent years, neural organoids derived from human pluripotent stem cells (hPSCs) have offered a transformative pre-clinical platform for understanding central nervous system (CNS) development, disease, drug effects, and toxicology. CNS vasculature plays an important role in all these scenarios; however, most published studies describe CNS organoids that lack a functional vasculature or demonstrate rudimentary incorporation of endothelial cells or blood vessel networks. Here, we review the existing knowledge of vascularization during the development of different CNS regions, including the brain, spinal cord, and retina, and compare it to vascularized CNS organoid models. We highlight several areas of contrast where further bioengineering innovation is needed and discuss potential applications of vascularized neural organoids in modeling human CNS development, physiology, and disease.
Keywords: CP: Stem cell research; angiogenesis; blood-brain barrier; central nervous system; endothelium; human pluripotent stem cell; microphysiological system; neural rosette; neuroepithelium; neurogenesis; neurovascular unit; organoid; perineural vascular plexus; periventricular plexus; vasculogenesis.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests R.S.A. is a co-owner of Neurosetta, LLC, which commercializes micropatterned neural rosette technology.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

