Loss of glymphatic homeostasis in heart failure
- PMID: 39693238
- PMCID: PMC11884761
- DOI: 10.1093/brain/awae411
Loss of glymphatic homeostasis in heart failure
Abstract
Heart failure is associated with progressive reduction in cerebral blood flow and neurodegenerative changes leading to cognitive decline. The glymphatic system is crucial for the brain's waste removal, and its dysfunction is linked to neurodegeneration. In this study, we used a mouse model of heart failure, induced by myocardial infarction, to investigate the effects of heart failure with reduced ejection fraction on the brain's glymphatic function. Using dynamic contrast-enhanced MRI and high-resolution fluorescence microscopy, we found increased solute influx from the CSF spaces to the brain, i.e. glymphatic influx, at 12 weeks post-myocardial infarction. Two-photon microscopy revealed that cerebral arterial pulsatility, a major driver of the glymphatic system, was potentiated at this time point, and could explain this increase in glymphatic influx. However, clearance of proteins from the brain parenchyma did not increase proportionately with influx, while a relative increase in brain parenchyma volume was found at 12 weeks post-myocardial infarction, suggesting dysregulation of brain fluid dynamics. Additionally, our results showed a correlation between brain clearance and cerebral blood flow. These findings highlight the role of cerebral blood flow as a key regulator of the glymphatic system, suggesting its involvement in the development of brain disorders associated with reduced cerebral blood flow. This study paves the way for future investigations into the effects of cardiovascular diseases on the brain's clearance mechanisms, which may provide novel insights into the prevention and treatment of cognitive decline.
Keywords: cardiovascular disease; cerebral blood flow; cerebrospinal fluid.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
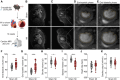
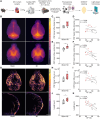



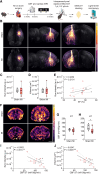
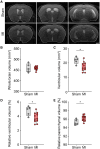
Comment in
-
From heart to head: glymphatic disruption as a new pathway in heart failure.Brain. 2025 Mar 6;148(3):695-697. doi: 10.1093/brain/awaf054. Brain. 2025. PMID: 39932877 No abstract available.
References
-
- Wu Y, Chen L, Zhong F, et al. Cognitive impairment in patients with heart failure: Molecular mechanism and therapy. Heart Fail Rev. 2023;28:807–820. - PubMed
-
- Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch Intern Med. 2006;166:1003–1008. - PubMed
-
- Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. - PubMed
-
- Cannon JA, Moffitt P, Perez-Moreno AC, et al. Cognitive impairment and heart failure: Systematic review and meta-analysis. J Card Fail. 2017;23:464–475. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

