A minimal complex of KHNYN and zinc-finger antiviral protein binds and degrades single-stranded RNA
- PMID: 39693345
- PMCID: PMC11670115
- DOI: 10.1073/pnas.2415048121
A minimal complex of KHNYN and zinc-finger antiviral protein binds and degrades single-stranded RNA
Abstract
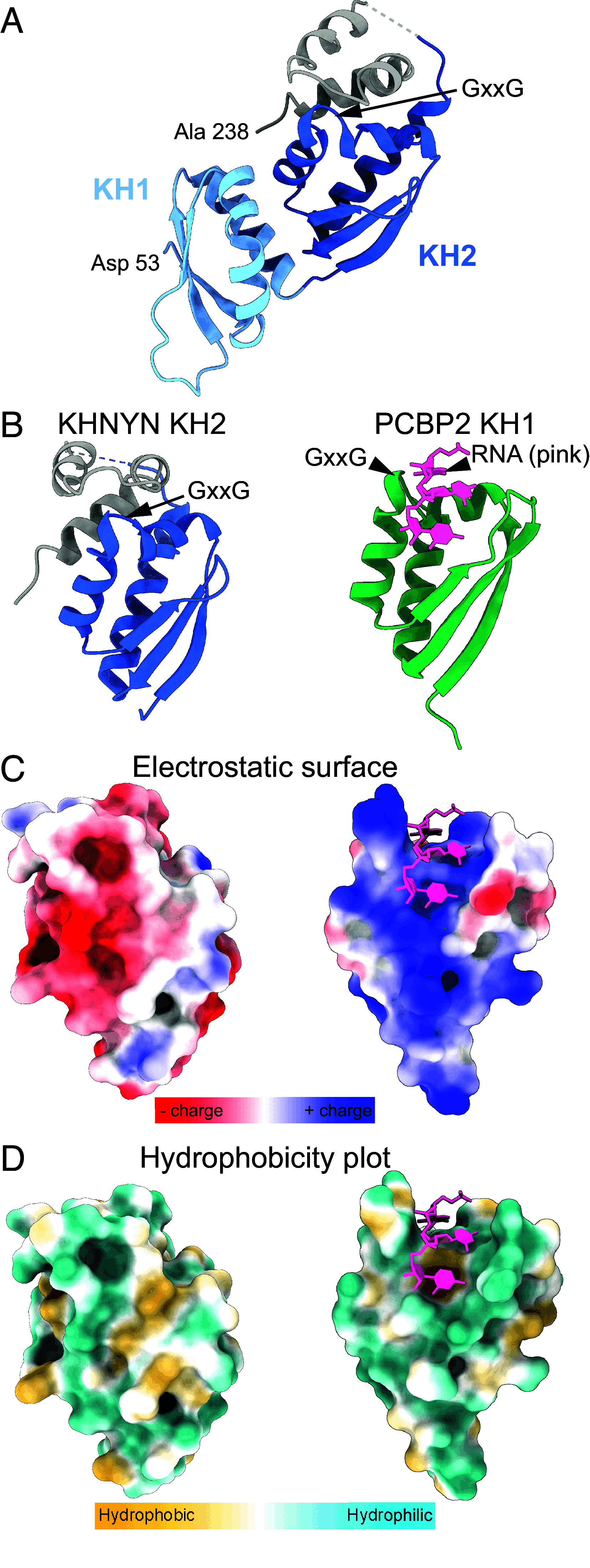
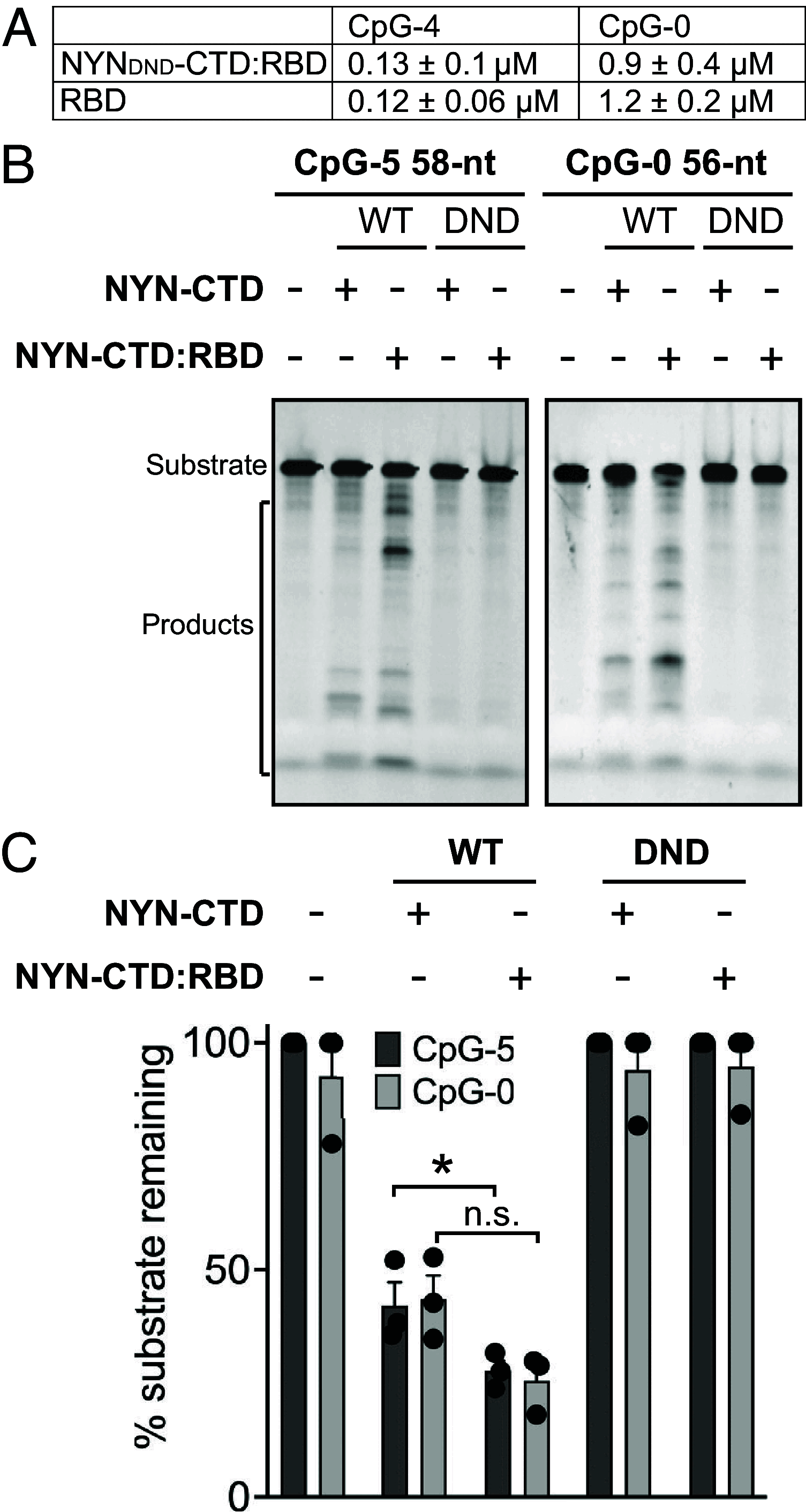
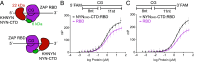
Detecting viral infection is a key role of the innate immune system. The genomes of some RNA viruses have a high CpG dinucleotide content relative to most vertebrate cell RNAs, making CpGs a molecular marker of infection. The human zinc-finger antiviral protein (ZAP) recognizes CpG, mediates clearance of the foreign CpG-rich RNA, and causes attenuation of CpG-rich RNA viruses. While ZAP binds RNA, it lacks enzymatic activity that might be responsible for RNA degradation and thus requires interacting cofactors for its function. One of these cofactors, KHNYN, has a predicted nuclease domain. Using biochemical approaches, we found that the KHNYN NYN domain is a single-stranded RNA ribonuclease that does not have sequence specificity and digests RNA with or without CpG dinucleotides equivalently in vitro. We show that unlike most KH domains, the KHNYN KH domain does not bind RNA. Indeed, a crystal structure of the KH region revealed a double-KH domain with a negatively charged surface that accounts for the lack of RNA binding. Rather, the KHNYN C-terminal domain (CTD) interacts with the ZAP RNA-binding domain (RBD) to provide target RNA specificity. We define a minimal complex composed of the ZAP RBD and the KHNYN NYN-CTD and use a fluorescence polarization assay to propose a model for how this complex interacts with a CpG dinucleotide-containing RNA. In the context of the cell, this module would represent the minimum ZAP and KHNYN domains required for CpG-recognition and ribonuclease activity essential for attenuation of viruses with clusters of CpG dinucleotides.
Keywords: RNA; anti-viral restriction; nuclease; protein crystal structure.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Gao G., Guo X., Goff S. P., Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science 297, 1703–1706 (2002). - PubMed
MeSH terms
Substances
Grants and funding
- P30GM138396/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- AC02-06CH11357/DOE | Office of Science (SC)
- ACB-12002/HHS | NIH | NCI | Center for Cancer Research (CCR)
- P30 GM138396/GM/NIGMS NIH HHS/United States
- AGM-12006/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources

