Immunopeptidomic MHC-I profiling and immunogenicity testing identifies Tcj2 as a new Chagas disease mRNA vaccine candidate
- PMID: 39693359
- PMCID: PMC11654963
- DOI: 10.1371/journal.ppat.1012764
Immunopeptidomic MHC-I profiling and immunogenicity testing identifies Tcj2 as a new Chagas disease mRNA vaccine candidate
Abstract
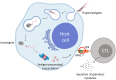
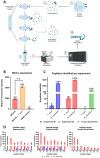
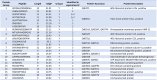
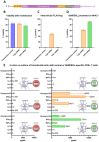
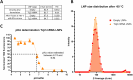
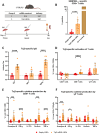
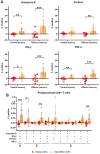
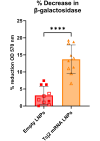
Trypanosoma cruzi is a protozoan parasite that causes Chagas disease. Globally 6 to 7 million people are infected by this parasite of which 20-30% will progress to develop Chronic Chagasic Cardiomyopathy (CCC). Despite its high disease burden, no clinically approved vaccine exists for the prevention or treatment of CCC. Developing vaccines that can stimulate T. cruzi-specific CD8+ cytotoxic T cells and eliminate infected cells requires targeting parasitic antigens presented on major histocompatibility complex-I (MHC-I) molecules. We utilized mass spectrometry-based immunopeptidomics to investigate which parasitic peptides are displayed on MHC-I of T. cruzi infected cells. Through duplicate experiments, we identified an array of unique peptides that could be traced back to 17 distinct T. cruzi proteins. Notably, six peptides were derived from Tcj2, a trypanosome chaperone protein and member of the DnaJ (heat shock protein 40) family, showcasing its potential as a viable candidate vaccine antigen with cytotoxic T cell inducing capacity. Upon testing Tcj2 as an mRNA vaccine candidate in mice, we observed a strong memory cytotoxic CD8+ T cell response along with a Th1-skewed humoral antibody response. In vitro co-cultures of T. cruzi infected cells with splenocytes of Tcj2-immunized mice restricted the replication of T. cruzi, demonstrating the protective potential of Tcj2 as a vaccine target. Moreover, antisera from Tcj2-vaccinated mice displayed no cross-reactivity with DnaJ in lysates from mouse and human indicating a decreased likelihood of triggering autoimmune reactions. Our findings highlight how immunopeptidomics can identify new vaccine targets for Chagas disease, with Tcj2 emerging as a promising new mRNA vaccine candidate.
Copyright: © 2024 Versteeg et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
LV, RA, JL, JW, NI, BK, MJV, CP, KJ, MEB, PH and JP collaborated in the development of Tc24-C4, a vaccine candidate against Chagas Disease that is currently undergoing clinical evaluation. JP, MEB and PH are listed among the inventors on a Chagas disease vaccine patent, submitted by Baylor College of Medicine.
Figures
References
-
- Lidani KCF, Andrade FA, Bavia L, Damasceno FS, Beltrame MH, Messias-Reason IJ, et al. Chagas Disease: From Discovery to a Worldwide Health Problem. Front Public Health [Internet]. 2019. [cited 2023 Apr 5];7. Available from: https://www.frontiersin.org/articles/10.3389/fpubh.2019.00166 - DOI - PMC - PubMed
-
- Pérez-Molina JA, Molina I. Chagas disease. The Lancet. 2018. Jan 6;391(10115):82–94. - PubMed
-
- Neglected Infectious Diseases in the Americas: Success Stories and Innovation to Reach the Neediest—PAHO/WHO | Pan American Health Organization [Internet]. [cited 2023 Apr 5]. https://www.paho.org/en/documents/neglected-infectious-diseases-americas...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

