An influenza mRNA vaccine protects ferrets from lethal infection with highly pathogenic avian influenza A(H5N1) virus
- PMID: 39693411
- PMCID: PMC12100637
- DOI: 10.1126/scitranslmed.ads1273
An influenza mRNA vaccine protects ferrets from lethal infection with highly pathogenic avian influenza A(H5N1) virus
Abstract
The global spread of the highly pathogenic avian influenza (HPAI) A(H5N1) virus poses a serious pandemic threat, necessitating the swift development of effective vaccines. The success of messenger RNA (mRNA) vaccine technology in the COVID-19 pandemic, marked by its rapid development and scalability, demonstrates its potential for addressing other infectious threats, such as HPAI A(H5N1). We therefore evaluated mRNA vaccine candidates targeting panzootic influenza A(H5) clade 2.3.4.4b viruses, which have been shown to infect a range of mammalian species, including most recently being detected in dairy cattle. Ferrets were immunized with mRNA vaccines encoding either hemagglutinin alone or hemagglutinin and neuraminidase, derived from a 2.3.4.4b prototype vaccine virus recommended by the World Health Organization. Kinetics of the immune responses, as well as protection against a lethal challenge dose of A(H5N1) virus, were assessed. Two doses of mRNA vaccination elicited robust neutralizing antibody titers against a 2022 avian isolate and a 2024 human isolate. Further, mRNA vaccination conferred protection from lethal challenge, whereas all unvaccinated ferrets succumbed to infection. It also reduced viral titers in the upper and lower respiratory tracts of infected ferrets. These results underscore the effectiveness of mRNA vaccines against HPAI A(H5N1), showcasing their potential as a vaccine platform for future influenza pandemics.
Conflict of interest statement
Figures


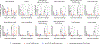
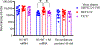
References
-
- Chan PK, Outbreak of avian influenza A(H5N1) virus infection in Hong Kong in 1997. Clin. Infect. Dis. 34, S58–S64 (2002). - PubMed
-
- World Health Organization Regional Office for the Western Pacific, “Human infection with avian influenza A(H5) viruses,” Avian Influenza Wkly. Update (no. 931) (26 January 2024).
-
- Nagy A, Černíková L, Stará M, A new clade 2.3.4.4b H5N1 highly pathogenic avian influenza genotype detected in Europe in 2021. Arch. Virol. 167, 1455–1459 (2022). - PubMed
-
- Bevins SN, Shriner SA, Cumbee JC Jr., K. E. Dilione, K. E. Douglass, J. W. Ellis, M. L. Killian, M. K. Torchetti, J. B. Lenoch, Intercontinental movement of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4 virus to the United States, 2021. Emerg. Infect. Dis. 28, 1006–1011 (2022). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

