Hydroalkylation of unactivated olefins with C(sp3)─H compounds enabled by NiH-catalyzed radical relay
- PMID: 39693419
- PMCID: PMC11654677
- DOI: 10.1126/sciadv.ads6885
Hydroalkylation of unactivated olefins with C(sp3)─H compounds enabled by NiH-catalyzed radical relay
Abstract
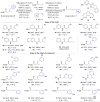
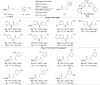
The hydroalkylation reaction of olefins with alkanes is a highly desirable synthetic transformation toward the construction of C(sp3)─C(sp3) bonds. However, such transformation has proven to be challenging for unactivated olefins, particularly when the substrates lack directing groups or acidic C(sp3)─H bonds. Here, we address this challenge by merging NiH-catalyzed radical relay strategy with a HAT (hydrogen atom transfer) process. In this catalytic system, a nucleophilic alkyl radical is generated from a C(sp3)─H compound in the presence of a HAT promotor, which couples with an alkyl metallic intermediate generated from the olefin substrate with a NiH catalyst to form the C(sp3)─C(sp3) bond. Starting from easily available materials, the reaction not only demonstrates wide functional group compatibility but also provides hydroalkylation products with regiodivergence and excellent enantioselectivity through effective catalyst control under mild conditions.
Figures


Similar articles
-
Methodologies for the synthesis of quaternary carbon centers via hydroalkylation of unactivated olefins: twenty years of advances.Beilstein J Org Chem. 2021 Jul 7;17:1565-1590. doi: 10.3762/bjoc.17.112. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34290837 Free PMC article. Review.
-
Hydroalkylation of Unactivated Olefins via Visible-Light-Driven Dual Hydrogen Atom Transfer Catalysis.J Am Chem Soc. 2021 Jul 28;143(29):11251-11261. doi: 10.1021/jacs.1c05852. Epub 2021 Jul 16. J Am Chem Soc. 2021. PMID: 34269582
-
Direct Construction of C-Alkyl Glycosides from Non-Activated Olefins via Nickel-Catalyzed C(sp3)─C(sp3) Coupling Reaction.Adv Sci (Weinh). 2024 Mar;11(12):e2307226. doi: 10.1002/advs.202307226. Epub 2024 Jan 18. Adv Sci (Weinh). 2024. PMID: 38235616 Free PMC article.
-
NiH-Catalyzed Reductive Relay Hydroalkylation: A Strategy for the Remote C(sp3 )-H Alkylation of Alkenes.Angew Chem Int Ed Engl. 2018 Apr 3;57(15):4058-4062. doi: 10.1002/anie.201712731. Epub 2018 Mar 13. Angew Chem Int Ed Engl. 2018. PMID: 29460343
-
N-Heterocyclic Carbene Enabled Functionalization of Inert C(Sp3)-H Bonds via Hydrogen Atom Transfer (HAT) Processes.Chemistry. 2024 Aug 22;30(47):e202401811. doi: 10.1002/chem.202401811. Epub 2024 Aug 2. Chemistry. 2024. PMID: 39092881 Review.
References
-
- Dong Z., Ren Z., Thompson S. J., Xu Y., Dong G., Transition-metal-catalyzed C─H alkylation using alkenes. Chem. Rev. 117, 9333–9403 (2017). - PubMed
-
- Fernández D. F., Mascarenas J. L., López F., Catalytic addition of C−H bonds across C−C unsaturated systems promoted by iridium(I) and its group IX congeners. Chem. Soc. Rev. 49, 7378–7405 (2020). - PubMed
-
- Woźniak Ł., Tan J.-F., Nguyen Q.-H., du Vigné A. M., Smal V., Cao Y.-X., Cramer N., Catalytic enantioselective functionalizations of C-H bonds by chiral iridium complexes. Chem. Rev. 120, 10516–10543 (2020). - PubMed
-
- DiPucchio R. C., Rosca S.-C., Schafer L. L., Hydroaminoalkylation for the catalytic addition of amines to alkenes or alkynes: Diverse mechanisms enable diverse substrate scope. J. Am. Chem. Soc. 144, 11459–11481 (2022). - PubMed
-
- Yi H., Zhang G., Wang H., Huang Z., Wang J., Singh A. K., Lei A., Recent advances in radical C-H activation/radical cross-coupling. Chem. Rev. 117, 9016–9085 (2017). - PubMed
LinkOut - more resources
Full Text Sources

