dCasMINI-mediated therapy rescues photoreceptors degeneration in a mouse model of retinitis pigmentosa
- PMID: 39693439
- PMCID: PMC11654696
- DOI: 10.1126/sciadv.adn7540
dCasMINI-mediated therapy rescues photoreceptors degeneration in a mouse model of retinitis pigmentosa
Abstract
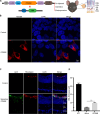
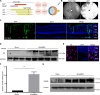
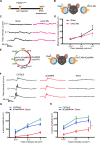
Retinitis pigmentosa (RP) is characterized by degeneration of rod and cone photoreceptors that progresses to irreversible blindness. Now, there are no mutation-agnostic approaches to treat RP. Here, we utilized a single adeno-associated virus (AAV)-based CRISPR activation system to activate phosphodiesterase 6B (Pde6b) to mitigate the severe degeneration in Pde6anmf363 mice. We demonstrate that transcriptional activation of Pde6b can rescue the loss of Pde6a, with preservation of retinal structure, restoration of electroretinography responses, and improvement of visual function as assessed by optokinetic response and looming-induced escape behaviors. These findings demonstrate the therapeutic potential of a dCasMINI-mediated activation strategy that provides a mutation-independent treatment for retinal degeneration. This study offers a promising therapeutic approach for RP and potentially other forms of genetic diseases.
Figures



References
-
- Hartong D. T., Berson E. L., Dryja T. P., Retinitis pigmentosa. Lancet 368, 1795–1809 (2006). - PubMed
-
- Kennan A., Aherne A., Humphries P., Light in retinitis pigmentosa. Trends Genet. 21, 103–110 (2005). - PubMed
-
- Streilein J. W., Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 3, 879–889 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

