Use of tenofovir-based preexposure prophylaxis among pregnant women in South Africa
- PMID: 39693489
- PMCID: PMC11902610
- DOI: 10.1097/QAD.0000000000004090
Use of tenofovir-based preexposure prophylaxis among pregnant women in South Africa
Abstract
Objective: We developed Healthy Families-PrEP to support perinatal women to use HIV prevention strategies.
Design: Single-arm study to evaluate PrEP use among pregnant women exposed to the intervention.
Methods: We offered safer conception counselling, including TDF/FTC as PrEP with adherence support (Healthy Families-PrEP) for women planning for pregnancy in South Africa with a partner with HIV or unknown serostatus. Women completed pregnancy and HIV testing quarterly and were followed for 1 year or until pregnancy end. For those initiating PrEP, electronic pillcap data and plasma were collected. We described PrEP adherence by proportion of days with pillcap openings and proportion of women with detected (≥10ng/ml) plasma tenofovir.
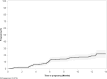
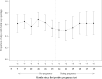
Results: From November 2017 to January 2020, 326 women with median age 24 years [interquartile range (IQR) 22-27] enrolled. Partner HIV-serostatus was unknown by 316 (97%). Over 3204 person-months of follow-up, 56 women became pregnant. Twenty-six women used PrEP during pregnancy and opened pillcaps on a mean of 53.1% [95% confidence interval (CI) 46.9-59.3%] of days. Plasma tenofovir was detected among 25, 15.4, and 12.5% of women providing samples during months 0-3, 4-6, and 7-9. No HIV seroconversions were observed.
Conclusion: We observed low-pregnancy incidence. Counselling may have encouraged delayed pregnancy plans; some women may have exaggerated pregnancy plans to enroll. About half of pregnant women used PrEP and took over half of doses by pillcap. Fewer than 25% had tenofovir detected, likely reflecting pregnancy-related pharmacokinetics and adherence challenges. High interest in pregnancy PrEP use highlights the need to optimize adherence support and prevention choice.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
L.T.M. has received operational support from Gilead Sciences. K.W.H. and K.B. are employed by and own equity in Target RWE, which has received fees from Amgen, Baxter International, Gilead Sciences, Janssen Research & Development (Janssen R&D), and Merck outside the submitted work. The remaining authors have no conflicts to declare.
Figures
References
-
- UNAIDS. Country Fact Sheets: South Africa (2021) 2022 (08 September 2022). Available at: https://www.unaids.org/en/regionscountries/countries/southafrica. [Accessed 1 February 2024].
-
- Thomson KA, Hughes J, Baeten JM, John-Stewart G, Celum C, Cohen CR, et al. Partners in Prevention HSV/HIV Transmission Study and Partners PrEP Study Teams. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis 2018; 218:16–25. - PMC - PubMed
-
- World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization 2021. - PubMed
-
- Woldesenbet SA, Lombard C, Manda S, Kufa T, Ayalew K, Cheyip M, Puren A. The 2019 National Antenatal Sentinel HIV Survey. National Department of Health, South Africa. 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous