Acute Promyelocytic Leukemia Asian Consortium study of arsenic trioxide in newly diagnosed patients: impact and outcome
- PMID: 39693517
- PMCID: PMC11875177
- DOI: 10.1182/bloodadvances.2024014999
Acute Promyelocytic Leukemia Asian Consortium study of arsenic trioxide in newly diagnosed patients: impact and outcome
Abstract
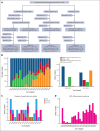
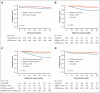
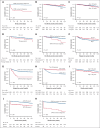
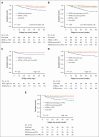
The Acute Promyelocytic Leukemia (APL) Asian Consortium analyzed a contemporaneous cohort of newly diagnosed patients with APL treated with and without frontline arsenic trioxide (ATO) in 6 centers. The objectives were to define the impact of ATO on early deaths and relapses and its optimal positioning in the overall treatment strategy. In a 21.5-year period, 324 males and 323 females at a median age of 45.5 years (range, 18.1-91.8; low/intermediate risk, n = 448; high risk, n = 199) were treated. Regimens included frontline all-trans retinoic acid (ATRA)/chemotherapy and maintenance with/without ATO (n = 436), ATRA/IV-ATO/chemotherapy (ATRA/IV-ATO; n = 61), and ATRA/oral-ATO/ascorbic acid with ATO maintenance (oral-AAA; n = 150). The ATRA/chemotherapy group had significantly more frequent early deaths within 60 days (8.3% vs 3.3%; P = .05), inferior 60-day survival (91.7% vs 98.4%/96%; P < .001), inferior 5-year relapse-free survival (RFS; 76.9% vs 92.8%/97.8%; P < .001), and inferior 5-year overall survival (OS; 84.6% vs 91.4%/92.3%; P = .03) than ATO-containing groups (ATRA/IV-ATO and oral-AAA). The addition of oral-ATO maintenance partly mitigated the inferior 5-year RFS resulting from the omission of ATO during induction (ATRA/chemotherapy/non-ATO maintenance vs ATRA/chemotherapy/ATO maintenance vs ATRA/IV-ATO vs oral-AAA, 71.1% vs 87.9% vs 92.8% vs 97.8%; P < .001). The favorable survival impacts of ATO were observed in all risk groups. In conclusion, ATO decreased early deaths, improved 60-day survival, and resulted in significantly superior RFS and OS. This trial was registered at www.clinicaltrials.gov as #NCT04251754.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: H.G. and Y.-L.K. are employees of the University of Hong Kong, which holds 2 US patents (7521071 B2 and 8906422 B2), 1 Japan patent (4786341), and 1 European patent (1562616 B1) for the use of oral-ATO in the treatment of leukemias and lymphomas. The remaining authors declare no competing financial interests.
Figures

References
-
- Tallman MS, Altman JK. How I treat acute promyelocytic leukemia. Blood. 2009;114(25):5126–5135. - PubMed
-
- Tallman MS, Andersen JW, Schiffer CA, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100(13):4298–4302. - PubMed
-
- Lengfelder E, Haferlach C, Saussele S, et al. High dose ara-C in the treatment of newly diagnosed acute promyelocytic leukemia: long-term results of the German AMLCG. Leukemia. 2009;23(12):2248–2258. - PubMed
-
- Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116(17):3171–3179. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

