Abemaciclib Plus Fulvestrant in Advanced Breast Cancer After Progression on CDK4/6 Inhibition: Results From the Phase III postMONARCH Trial
- PMID: 39693591
- PMCID: PMC11936477
- DOI: 10.1200/JCO-24-02086
Abemaciclib Plus Fulvestrant in Advanced Breast Cancer After Progression on CDK4/6 Inhibition: Results From the Phase III postMONARCH Trial
Abstract
Purpose: Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) combined with endocrine therapy (ET) are the standard first-line treatment for hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC); however, disease progression occurs in almost all patients and additional treatment options are needed. Herein, we report outcomes of the postMONARCH trial investigating a switch in ET with/without CDK4/6 inhibition with abemaciclib after disease progression on CDK4/6i.
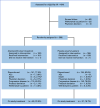
Methods: This double-blind, randomized phase III study enrolled patients with disease progression on previous CDK4/6i plus aromatase inhibitor as initial therapy for advanced disease or recurrence on/after adjuvant CDK4/6i + ET. Patients were randomly assigned (1:1) to abemaciclib + fulvestrant or placebo + fulvestrant. The primary end point was investigator-assessed progression-free survival (PFS). Secondary end points included PFS by blinded independent central review, objective response rate (ORR), and safety.
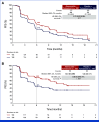
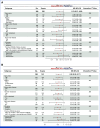
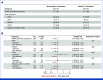
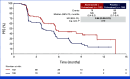
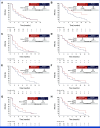
Results: This study randomly assigned 368 patients (abemaciclib + fulvestrant, n = 182 placebo + fulvestrant, n = 186). At the primary analysis (258 events), the hazard ratio (HR) was 0.73 (95% CI, 0.57 to 0.95; nominal P = .017), with median PFS 6.0 (95% CI, 5.6 to 8.6) versus 5.3 (95% CI, 3.7 to 5.6) months and 6-month PFS rates of 50% and 37% in the abemaciclib + fulvestrant and placebo + fulvestrant arms, respectively. These results were supported by BICR-assessed PFS (HR, 0.55 [95% CI, 0.39 to 0.77]; nominal P < .001). A consistent treatment effect was seen across major clinical and genomic subgroups, including with/without ESR1 or PIK3CA mutations. Among patients with measurable disease, investigator-assessed ORR was improved with abemaciclib + fulvestrant versus placebo + fulvestrant (17% v 7%; nominal P = .015). No new safety signals were observed, with findings consistent with the known safety profile of abemaciclib.
Conclusion: Abemaciclib + fulvestrant significantly improved PFS after disease progression on previous CDK4/6i + ET in patients with HR+, HER2- ABC, offering an additional targeted therapy option for these patients.
Trial registration: ClinicalTrials.gov NCT05169567.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
This author is a member of the
No other potential conflicts of interest were reported.
Figures
References
-
- Gradishar WJ, Moran MS, Abraham J, et al. : NCCN Guidelines® Insights: Breast cancer, version 4.2023. J Natl Compr Cancer Netw 21:594-608, 2023 - PubMed
-
- Patnaik A, Rosen LS, Tolaney SM, et al. : Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other Solid tumors. Cancer Discov 6:740-753, 2016 - PubMed
-
- Johnston SRD, Toi M, O'Shaughnessy J, et al. : Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol 24:77-90, 2023 - PMC - PubMed
-
- Rastogi P, O'Shaughnessy J, Martin M, et al. : Adjuvant abemaciclib plus endocrine therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative, high-risk early breast cancer: Results from a preplanned monarchE overall survival interim analysis, including 5-year efficacy outcomes. J Clin Oncol 42:987-993, 2024 - PMC - PubMed
-
- Goetz MP, Toi M, Huober J, et al. : Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2- advanced breast cancer: Final overall survival results of MONARCH 3. Ann Oncol 35:718-727, 2024 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

