Antibody-Drug Conjugates Targeting the EGFR Ligand Epiregulin Elicit Robust Antitumor Activity in Colorectal Cancer
- PMID: 39693606
- PMCID: PMC11875910
- DOI: 10.1158/0008-5472.CAN-24-0798
Antibody-Drug Conjugates Targeting the EGFR Ligand Epiregulin Elicit Robust Antitumor Activity in Colorectal Cancer
Abstract
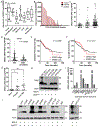
As colorectal cancer remains a leading cause of cancer-related death, identifying therapeutic targets and approaches is essential to improve patient outcomes. The EGFR ligand epiregulin (EREG) is highly expressed in RAS wild-type (WT) and mutant colorectal cancer, with minimal expression in normal tissues, making it an attractive target for antibody-drug conjugate (ADC) development. In this study, we produced and purified an EREG mAb, H231, which had high specificity and affinity for human and mouse EREG. H231 also internalized to lysosomes, which is important for ADC payload release. ImmunoPET and ex vivo biodistribution studies showed significant tumor uptake of zirconium-89-labeled H231, with minimal uptake in normal tissues. H231 was conjugated to either cleavable dipeptide or tripeptide chemical linkers attached to the DNA-alkylating payload duocarmycin DM, and the cytotoxicity of EREG ADCs was assessed in a panel of colorectal cancer cell lines. EREG ADCs incorporating tripeptide linkers demonstrated the highest potency in EREG-expressing colorectal cancer cells irrespective of RAS mutations. Preclinical safety and efficacy studies showed that EREG ADCs were well tolerated, neutralized EGFR pathway activity, caused significant tumor growth inhibition or regression, and increased survival in colorectal cancer cell line and patient-derived xenograft models. These data suggest that EREG is a promising target for the development of ADCs for treating colorectal cancer and other cancer types that express high levels of EREG. Although the efficacy of clinically approved anti-EGFR mAbs is largely limited by RAS mutational status, EREG ADCs may show promise for both RAS mutant and WT patients, thus improving existing treatment options. Significance: EREG-targeting antibody-drug conjugates demonstrate acceptable safety and robust therapeutic efficacy in RAS mutant and wild-type colorectal cancer, suggesting their potential as an alternative to EGFR-targeted therapy to benefit a broader patient population.
©2024 American Association for Cancer Research.
Conflict of interest statement
Figures


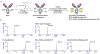


Update of
-
Antibody-Drug Conjugates Targeting the EGFR Ligand Epiregulin Elicit Robust Anti-Tumor Activity in Colorectal Cancer.bioRxiv [Preprint]. 2024 Nov 21:2024.02.20.581056. doi: 10.1101/2024.02.20.581056. bioRxiv. 2024. Update in: Cancer Res. 2025 Mar 03;85(5):973-986. doi: 10.1158/0008-5472.CAN-24-0798. PMID: 39605519 Free PMC article. Updated. Preprint.
References
-
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73:233–54 - PubMed
-
- Fakih MG, Salvatore L, Esaki T, Modest DP, Lopez-Bravo DP, Taieb J, et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N Engl J Med 2023;389:2125–39 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

