Oral inflammation and microbiome dysbiosis exacerbate chronic graft-versus-host disease
- PMID: 39693612
- PMCID: PMC11867138
- DOI: 10.1182/blood.2024024540
Oral inflammation and microbiome dysbiosis exacerbate chronic graft-versus-host disease
Abstract
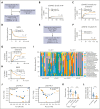
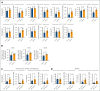
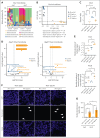
The oral microbiota, second in abundance to the gut, is implicated in chronic systemic diseases, but its specific role in graft-versus-host disease (GVHD) pathogenesis has been unclear. Our study finds that mucositis-induced oral dysbiosis in patients after hematopoietic cell transplantation (HCT) associated with increased chronic GVHD (cGVHD), even in patients receiving posttransplant cyclophosphamide. In murine HCT models, oral dysbiosis caused by bilateral molar ligatures exacerbated cGVHD and increased bacterial load in the oral cavity and gut, with Enterococcaceae significantly increasing in both organs. In this model, the migration of Enterococcaceae to cervical lymph nodes both before and after transplantation activated antigen-presenting cells, thereby promoting the expansion of donor-derived inflammatory T cells. Based on these results, we hypothesize that pathogenic bacteria increase in the oral cavity might not only exacerbate local inflammation but also enhance systemic inflammation throughout the HCT course. Additionally, these bacteria translocated to the gut and formed ectopic colonies, further amplifying systemic inflammation. Furthermore, interventions targeting the oral microbiome mitigated murine cGVHD. Collectively, our findings highlight the importance of oral dysbiosis in cGVHD and suggest that modulation of the oral microbiome during transplantation may be an effective approach for preventing or treating cGVHD.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: N.A. reports research funding from Novartis, Japan and honoraria from Novartis, Japan; AbbVie, Japan; Astellas, Japan; Asahi Kasei, Japan; Chugai, Japan; and Otsuka, Japan. D.E. reports research funding from Nippon Shinyaku, Japan; Chugai, Japan; and Otsuka, Japan; and honoraria from Eisai, Japan; Chugai, Japan; Kyowa Kirin, Japan; SymBio, Japan; and Bristol Myers Squibb, Japan. The remaining authors declare no competing financial interests.
Figures




Comment in
-
Appreciating the oral microbiome's impact on GVHD.Blood. 2025 Feb 20;145(8):791-793. doi: 10.1182/blood.2024027670. Blood. 2025. PMID: 39976948 No abstract available.
References
-
- Jenq RR, van den Brink MRM. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010;10(3):213–221. - PubMed
-
- Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105(5):2227–2234. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

