A dataset profiling the multiomic landscape of the prefrontal cortex in amyotrophic lateral sclerosis
- PMID: 39693632
- PMCID: PMC11653894
- DOI: 10.1093/gigascience/giae100
A dataset profiling the multiomic landscape of the prefrontal cortex in amyotrophic lateral sclerosis
Abstract
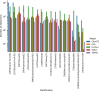
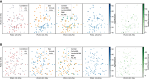
Amyotrophic lateral sclerosis (ALS) is the most common motor neuron disease, which still lacks effective disease-modifying therapies. Similar to other neurodegenerative disorders, such as Alzheimer and Parkinson disease, ALS pathology is presumed to propagate over time, originating from the motor cortex and spreading to other cortical regions. Exploring early disease stages is crucial to understand the causative molecular changes underlying the pathology. For this, we sampled human postmortem prefrontal cortex (PFC) tissue from Brodmann area 6, an area that exhibits only moderate pathology at the time of death, and performed a multiomic analysis of 51 patients with sporadic ALS and 50 control subjects. To compare sporadic disease to genetic ALS, we additionally analyzed PFC tissue from 4 transgenic ALS mouse models (C9orf72-, SOD1-, TDP-43-, and FUS-ALS) using the same methods. This multiomic data resource includes transcriptome, small RNAome, and proteome data from female and male samples, aimed at elucidating early and sex-specific ALS mechanisms, biomarkers, and drug targets.
Keywords: amyotrophic lateral sclerosis; early disease mechanisms; multiomics analysis; neurodegeneration; prefrontal cortex.
© The Author(s) 2024. Published by Oxford University Press GigaScience.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

