Distinct effects of obesity and diabetes on the action potential waveform and inward currents in rat ventricular myocytes
- PMID: 39693640
- PMCID: PMC12203996
- DOI: 10.1042/CS20242144
Distinct effects of obesity and diabetes on the action potential waveform and inward currents in rat ventricular myocytes
Abstract
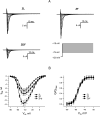
Obesity is a significant global health challenge, increasing the risk of developing type 2 diabetes mellitus (T2DM) and cardiovascular disease. Research indicates that obese individuals, regardless of their diabetic status, have an increased risk of cardiovascular complications. Studies suggest that these patients experience impaired electrical conduction in the heart, although the underlying cause-whether due to obesity-induced fat toxicity or diabetes-related factors-remains uncertain. This study investigated ventricular action potential parameters, as well as sodium (INa) and calcium (ICa, L) currents, in Zucker fatty (ZF) rats and Zucker diabetic fatty (ZDF) rats, which serve as models for obesity and T2DM, respectively. Ventricular myocytes were isolated from 25- to 30-week-old Zucker rats. Resting and action potentials were recorded using a β-escin perforated patch clamp, while INa and ICa,L were assessed with whole-cell patch clamp methods. ZF rats exhibited higher excitability and faster upstroke velocity with greater INa density, whereas ZDF rats showed decreased INa and slower action potential upstroke. No differences in ICa,L density or voltage sensitivity were found among the groups. In summary, obesity, with or without accompanying T2DM, distinctly impacts the action potential waveform, INa density, and excitability of ventricular myocytes in this rat model of T2DM.
Keywords: Zucker rat; action potential; type 2 diabetes; voltage-gated Ca2+current; voltage-gated Na+current.
© 2025 The Author(s); published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscrip
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

