Pharmacologic background and clinical issue of anti-influenza drugs
- PMID: 39694499
- PMCID: PMC11799661
- DOI: 10.5387/fms.24-00029
Pharmacologic background and clinical issue of anti-influenza drugs
Abstract
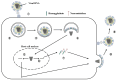
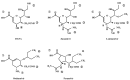
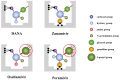
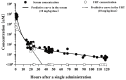
Since 2000, rapid antigen detection kits and anti-influenza drugs have been used for the early diagnosis and treatment of influenza in Japan, respectively. The main drugs available in clinical practice are the neuraminidase inhibitors oseltamivir, zanamivir, laninamivir, and peramivir, as well as the cap-dependent endonuclease inhibitor baloxavir marboxil. Antiviral therapy with neuraminidase inhibitors has been practiced for many years, especially in Japan; it can shorten the febrile period and reduce complications. Despite having similar structures, the pharmacologic background of neuraminidase inhibitors differs significantly, as reflected in their varying clinical efficacy. Due to its inhibitory mechanism, baloxavir marboxil can rapidly reduce the viral load than neuraminidase inhibitors. However, the duration of symptoms was similar after the administration of baloxavir marboxil and oseltamivir, and variants with reduced drug susceptibility have been detected in 20%-30% of pediatric patients treated with baloxavir marboxil. Clinical trials of several novel anti-influenza drugs are currently underway. When these drugs are first marketed, the characteristics of the influenza virus and the pharmacologic background of the drugs must be clarified before their administration to patients in clinical practice.
Keywords: anti-influenza drug; antiviral; children; influenza.
Conflict of interest statement
I declare no personal conflicts of interest.
Figures


References
-
- Wright PF, Neumann G, Kawaoka Y: Orthomyxoviruses. In Knipe DM, Howley PM, eds: Fields Virology. 6th ed. Lippincott Williams & Wilkins, Philadelphia, 1186-1243, 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

