Intestinal barrier function declines during polycystic kidney disease progression
- PMID: 39694536
- PMCID: PMC12176450
- DOI: 10.1152/ajprenal.00058.2024
Intestinal barrier function declines during polycystic kidney disease progression
Abstract
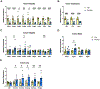
Most patients with autosomal dominant polycystic kidney disease (ADPKD) develop kidney cysts due to germline PKD1 mutations. In the kidney, Pkd1 loss impairs epithelial cell integrity and increases macrophage infiltration, contributing to cyst growth. Despite its role as the body's largest inflammatory cell reservoir, it has yet to be elucidated whether a similar phenotype presents in the intestines. We hypothesize that loss of Pkd1 leads to a leaky intestinal epithelial barrier and increased inflammation, before rapid cystogenesis. Control and inducible, global Pkd1 knockout (Pkd1KO) mice were euthanized at 3 and 6 mo of age (early and late stage) to evaluate kidney disease progression, small and large intestinal integrity, and inflammation. Early-stage Pkd1KO mice displayed mild cystic kidneys and tubular injury with preserved kidney function. Intestinal epithelial barrier was tighter in KO mice, which was associated with higher expression of cell-cell epithelial integrity markers. However, there was no evidence of local or systemic inflammation in either genotype. Late-stage Pkd1KO mice had severely cystic, impaired kidneys with increased expression of integrity markers, tubular injury, and inflammation. Intestinal epithelial barrier was leakier in late-stage Pkd1KO mice, accompanied by gene reduction of integrity markers, increased inflammation, and elevated water and sodium channel expression. Gut motility and fecal water excretion were increased in Pkd1KO compared with flox mice irrespective of age. Overall, kidney injury appears to precede intestinal injury in ADPKD, whereby the intestinal barrier becomes leaky as cystogenesis progresses.NEW & NOTEWORTHY Though autosomal dominant polycystic kidney disease (ADPKD) is a multisystem disorder, this is the first study to explore a kidney-gut contribution to disease progression. We identified a tightened intestinal epithelial barrier in early PKD, which becomes leaky as kidneys become more cystic, accompanied by a sustained loss of fecal water. Given the only approved ADPKD therapeutic yields adverse aquaretic events, this study emphasizes the need to evaluate extrarenal water loss in patients before prescribing.
Keywords: ADPKD; colon; integrity; motility; water.
Copyright © 2025 The Authors.
Conflict of interest statement
DISCLOSURES
The authors have no conflicts of interest to disclose.
Figures
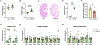

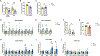
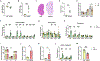



References
-
- The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. The European Polycystic Kidney Disease Consortium. Cell 78: 725, 1994. - PubMed
-
- Boletta A, Qian F, Onuchic LF, Bragonzi A, Cortese M, Deen PM, Courtoy PJ, Soria MR, Devuyst O, Monaco L, and Germino GG. Biochemical characterization of bona fide polycystin-1 in vitro and in vivo. Am J Kidney Dis 38: 1421–1429, 2001. - PubMed
-
- Russo RJ, Husson H, Joly D, Bukanov NO, Patey N, Knebelmann B, and Ibraghimov-Beskrovnaya O. Impaired formation of desmosomal junctions in ADPKD epithelia. Histochemistry and Cell Biology 124: 487–497, 2005. - PubMed
-
- Natoli TA, Gareski TC, Dackowski WR, Smith L, Bukanov NO, Russo RJ, Husson H, Matthews D, Piepenhagen P, and Ibraghimov-Beskrovnaya O. Pkd1 and Nek8 mutations affect cell-cell adhesion and cilia in cysts formed in kidney organ cultures. American Journal of Physiology-Renal Physiology 294: F73–F83, 2008. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

