USP33 Regulates DNA Damage Response and Carcinogenesis Through Deubiquitylating and Stabilising p53
- PMID: 39694539
- PMCID: PMC12099211
- DOI: 10.1111/cpr.13793
USP33 Regulates DNA Damage Response and Carcinogenesis Through Deubiquitylating and Stabilising p53
Abstract
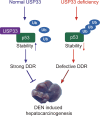
The de-ubiquitinase USP33 has been shown to possess either tumour-promoting or inhibitory effect on human cancer cells. However, all these findings are mainly based on in vitro cell culture models, and the in vivo evidence, which is more plausible to digest the functional role of USP33 in carcinogenic process, is still lacking. Here, we demonstrate that USP33 modulates DNA damage responses including cell cycle arrest and apoptosis induction through associating with p53. It directly interacts with p53 to mediate its de-ubiquitination and further stabilisation under DNA damage condition. Depletion of USP33 induces an enhanced level of p53 ubiquitination, which de-stabilises p53 protein leading to impaired DNA damage responses. Furthermore, USP33 silencing shows either promoted or inhibited effect on cell proliferation in human cancer cells with p53 WT and mutant background, respectively. Consistently, mice with hepatocyte-specific USP33 knockout are more sensitive to nitrosodiethylamine (DEN)-induced hepatocarcinogenesis compared to wild type mice. Thus, our in vitro and in vivo evidences illustrate that USP33 possesses anti-tumour activity via regulating p53 stability and activity.
© 2024 The Author(s). Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Chen J., Shan W., Jia Q., et al., “USP33 Facilitates the Ovarian Cancer Progression via Deubiquitinating and Stabilizing CBX2,” Oncogene 43 (2024): 3170–3183. - PubMed
MeSH terms
Substances
Grants and funding
- 2019YFA0801702/National Key R&D Program of China
- 32121001/the Innovative Research Group Program of National Science Foundation of China
- 2021096/Youth Innovation Promotion Association Foundation of Chinese Academy of Sciences of China
- XDA0460403/the Strategic Priority Research Program of the Chinese Academy of Sciences
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

