The epithelial era of asthma research: knowledge gaps and future direction for patient care
- PMID: 39694589
- PMCID: PMC11653196
- DOI: 10.1183/16000617.0221-2024
The epithelial era of asthma research: knowledge gaps and future direction for patient care
Abstract
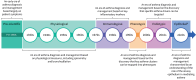
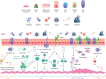
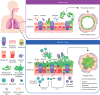
The Epithelial Science Expert Group convened on 18-19 October 2023, in Naples, Italy, to discuss the current understanding of the fundamental role of the airway epithelium in asthma and other respiratory diseases and to explore the future direction of patient care. This review summarises the key concepts and research questions that were raised. As an introduction to the epithelial era of research, the evolution of asthma management throughout the ages was discussed and the role of the epithelium as an immune-functioning organ was elucidated. The role of the bronchial epithelial cells in lower airway diseases beyond severe asthma was considered, as well as the role of the epithelium in upper airway diseases such as chronic rhinosinusitis. The biology and application of biomarkers in patient care was also discussed. The Epithelial Science Expert Group also explored future research needs by identifying the current knowledge and research gaps in asthma management and ranking them by priority. It was identified that there is a need to define and support early assessment of asthma to characterise patients at high risk of severe asthma. Furthermore, a better understanding of asthma progression is required. The development of new treatments and diagnostic tests as well as the identification of new biomarkers will also be required to address the current unmet needs. Finally, an increased understanding of epithelial dysfunction will determine if we can alter disease progression and achieve clinical remission.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: C.E. Brightling has received grants and consultancy fees from 4D Pharma, Areteia, AstraZeneca, Chiesi, Genentech, GSK, Mologic, Novartis, Regeneron Pharmaceuticals, Roche and Sanofi. G. Marone has received consulting fees from AstraZeneca. H. Aegerter has received fees from AstraZeneca and speaker fees from GSK. P. Chanez has received consultancy fees from AstraZeneca, Boehringer Ingelheim, Boston Scientific, Chiesi, GSK, Novartis, Sanofi, SNCF and Teva Pharmaceuticals; has received fees for advisory boards from AB Science, ALK, Argenx, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Sanofi and Teva Pharmaceuticals; has received fees from ALK, AstraZeneca, Boehringer Ingelheim, Boston Scientific, Chiesi, GSK, Novartis and Teva Pharmaceuticals; and has received grants from ALK, AstraZeneca, Boehringer Ingelheim, Boston Scientific, Novartis and Roche. E. Heffler has received speaker fees from Almirall, AstraZeneca, Celltrion, Chiesi, GSK, Novartis, Regeneron Pharmaceuticals, Sanofi and Stallergenes Greer. I.D. Pavord has received speaker's fees from Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Sanofi and Regeneron Pharmaceuticals; has received fees for attending advisory panels from Almirall, AstraZeneca, Boehringer Ingelheim, Dey Pharma, GSK, MSD, Napp Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, Sanofi and Schering-Plough; and has received sponsorship from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Napp Pharmaceuticals. K.F. Rabe has received fees for lectures, presentations, manuscript writing, attendance at speakers’ bureaus, and educational events from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GSK, Novartis, Regeneron Pharmaceuticals, Roche Pharma and Sanofi; and has received fees for advisory board participation from AstraZeneca, Boehringer Ingelheim, Regeneron Pharmaceuticals and Sanofi. L. Uller has received fees for activities within AstraZeneca Nordic; is part of the UPSTEAM investigator-driven study and has received research funding for Epitez explorative study. D. Dorscheid is supported by the following grants and clinical trials: AstraZeneca, British Columbia Lung Association, Canadian Institutes of Health Research, Michael Smith Foundation for Health Research, Teva Pharmaceuticals and Sanofi Regeneron. He has received speaking fees, travel grants, unrestricted project grants and writing fees and is a paid consultant via ad boards and other mechanisms for AstraZeneca, GSK, Novartis Canada, Sanofi Regeneron and Valeo Pharma.
Figures

References
-
- Asthma.net . History of asthma (part one): in the beginning. Date last accessed: 10 November 2023. Date last updated: 10 July 2017. https://asthma.net/living/history-of-asthma-part-one-in-the-beginning
-
- Rackemann FM. A clinical study of one hundred and fifty cases of bronchial asthma. Arch Intern Med 1918; XXII: 517–552. doi: 10.1001/archinte.1918.00090150111007 - DOI
-
- Subcommittee on Clinical Trials in Asthma . CONTROLLED trial of effects of cortisone acetate in status asthmaticus. Lancet 1956; 271: 803–806. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
