Cost-effectiveness of integrating paediatric tuberculosis services into child healthcare services in Africa: a modelling analysis of a cluster-randomised trial
- PMID: 39694623
- PMCID: PMC11667313
- DOI: 10.1136/bmjgh-2024-016416
Cost-effectiveness of integrating paediatric tuberculosis services into child healthcare services in Africa: a modelling analysis of a cluster-randomised trial
Abstract
Background: In 2021, over one million children developed tuberculosis, resulting in 214 000 deaths, largely due to inadequate diagnosis and treatment. The diagnosis and treatment of tuberculosis is limited in most high-burden countries because services are highly centralised at secondary/tertiary levels and are managed in a vertical, non-integrated way. To improve case detection and treatment among children, the World Health Organisation (WHO) recommends decentralised and integrated tuberculosis care models. The Integrating Paediatric TB Services Into Child Healthcare Services in Africa (INPUT) stepped-wedge cluster-randomised trial evaluated the impact of integrating tuberculosis services into healthcare for children under five in Cameroon and Kenya, compared with usual care, finding a 10-fold increase in tuberculosis case detection in Cameroon but no effect in Kenya.
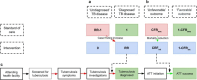
Methods: We estimated intervention impact on healthcare outcomes, resource use, health system costs and cost-effectiveness relative to the standard of care (SoC) using a decision tree analytical approach and data from the INPUT trial. INPUT trial data on cascades, resource use and intervention diagnostic rate ratios were used to parametrise the decision tree model. Health outcomes following tuberculosis treatment were modelled in terms of mortality and disability-adjusted life-years (DALYs).
Findings: For every 100 children starting antituberculosis treatment under SoC, an additional 876 (95% uncertainty interval (UI) -76 to 5518) in Cameroon and -6 (95% UI -61 to 96) in Kenya would start treatment under the intervention. Treatment success would increase by 5% in Cameroon and 9% in Kenya under the intervention compared with SoC. An estimated 350 (95% UI -31 to 2204) and 3 (95% UI -22 to 48) deaths would be prevented in Cameroon and Kenya, respectively. The incremental cost-effectiveness ratio for the intervention compared with SoC was US$506 and US$1299 per DALY averted in Cameroon and Kenya, respectively.
Interpretation: Although likely to be effective, the cost-effectiveness of integrating tuberculosis services into child healthcare services depends on baseline service coverage, tuberculosis detection and treatment outcomes.
Keywords: Health economics; Mathematical modelling; Paediatrics; Treatment; Tuberculosis.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- World Health Organization . Geneva: World Health Organization; [15-Jul-2024]. Global tuberculosis report 2023.https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa... Available. accessed.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
