What is the true target population for biologics in real-life COPD or asthma-COPD overlap patients?
- PMID: 39694677
- PMCID: PMC11667443
- DOI: 10.1136/bmjresp-2024-002702
What is the true target population for biologics in real-life COPD or asthma-COPD overlap patients?
Abstract
Introduction: Biologics provide significant benefits in asthma, reducing exacerbations and symptoms. Some biologics have shown promising results in small subgroups of patients with chronic obstructive pulmonary disease (COPD) and frequent exacerbations. Nevertheless, real-life data on the size of the COPD target population remain scarce.
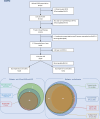
Methods: We analysed the characteristics of COPD and coexisting asthma and COPD patients included in the prospective multicentre, French COhort of BRonchial obstruction and Asthma, between 2008 and 2023 and evaluated the number of patients who could correspond to the inclusion criteria of randomised controlled trials evaluating various biologics targeting interleukin 33 (IL-33) (-receptor), IL-5 (-receptor), IL-4Rα or TSLP, in routine clinical practice.
Results: Among 434 COPD patients, only 21.7% met inclusion criteria for at least one biologic. Among patients with asthma, 54 (3.5%) had coexisting features of COPD in terms of age, smoking status and airflow obstruction and met inclusion criteria for at least one biologic. Notably, these patients were predominantly female, with worse lung function. Globally, the target chronic airway diseases population of the eagerly awaited biologics remains limited to a small part (ie, 1.3%-8%, depending on the biologic).
Conclusion: In a real-life COPD and asthma population (including asthmatic patients with features of COPD), the proportion of patients satisfying selection criteria applied in randomised controlled trials assessing the efficacy of biologics remains limited to less than 10% of the whole population.
Keywords: Asthma Pharmacology; Asthma in primary care; COPD Pharmacology; Eosinophil Biology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MZ reports grants and personal fees from Menarini, personal fees from Sanofi, personal fees from Chiesi, personal fees from AstraZeneca, personal fees from CSLBehring and personal fees from GSK outside the submitted work, grants from AVAD, grants from FRM. FH has no conflict of interest to declare related to the present work. LG has no conflict of interest to declare related to the present work. He received grants from AADAIRC. He received speaker or advisory board fees from GSK, ASTEN, Air Liquide Medical System, Sanofi Genzyme, SOS Oxygene, AstraZeneca, ASV, Boerhinger, VIVISOL and RESMED. CT has no conflict of interest to declare related to the present work. She received grants from GSK to INSERM UMR 1152. She received speaker or advisory board fees from AstraZeneca, GSK, Novartis, Sanofi, Stallergenes, Leo Pharma outside the submitted work. JG-B has no conflict of interest to declare related to the present work. MG has no conflicts of interest to disclose. NR has no conflict of interest to declare related to the present work. He received grants from Boehringer Ingelheim, Novartis, GSK and Pfizer to Institution. He received speaker or advisory board fees from Boehringer Ingelheim, AstraZeneca, GSK, Novartis, Sanofi, Chiesi, Pfizer, Novartis, Teva, Bayer, Austral, Biosency, Zambon, MSD, Menarini outside the submitted work. AP has no conflict of interest to declare related to the present work. P-OG has no conflict of interest to declare related to the present work. He received grants from AstraZeneca. He received speaker or advisory board fees from AstraZeneca, GSK, Sanofi, Chiesi, outside the submitted work. AB has no conflict of interest to declare related to the present work. NM has no conflict of interest to declare related to the present work. PB reports grants and personal fees from AstraZeneca, Menarini, personal fees from Sanofi, personal fees from Chiesi, grants from AVAD, grants from FRM, grants from AstraZeneca.
Figures

References
-
- GINA Reports. Glob. Initiat. Asthma. https://ginasthma.org/reports Available.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
