Blocking IL1RAP on cancer-associated fibroblasts in pancreatic ductal adenocarcinoma suppresses IL-1-induced neutrophil recruitment
- PMID: 39694705
- PMCID: PMC11660328
- DOI: 10.1136/jitc-2024-009523
Blocking IL1RAP on cancer-associated fibroblasts in pancreatic ductal adenocarcinoma suppresses IL-1-induced neutrophil recruitment
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) represents a major clinical challenge due to its tumor microenvironment, which exhibits immune-suppressive properties that facilitate cancer progression, metastasis, and therapy resistance. Interleukin 1 (IL-1) signaling has been implicated as a driver in this process. Mechanistically, both IL-1α and IL-1β bind to the IL-1 receptor type 1, forming a complex with IL-1-receptor accessory protein (IL1RAP), which triggers downstream signaling pathways. The IL1RAP blocking antibody nadunolimab is currently in clinical development, but the precise consequences of inhibiting IL-1 signaling in PDAC remains elusive.
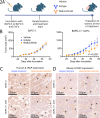
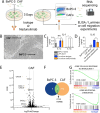
Methods: To evaluate the biological relevance of blocking IL1RAP using nadunolimab in a PDAC animal model, human PDAC cells and cancer-associated fibroblasts (CAFs) were co-transplanted into mice. To study the underlying mechanisms of IL1RAP blockade ex vivo, co-cultured PDAC cells and CAFs were treated with nadunolimab prior to RNA sequencing. Migration assays were performed to assess how nadunolimab affects interactions between CAFs and myeloid immune cells. Finally, to establish a clinical correlation between IL1RAP expression and nadunolimab treatment effects, we analyzed tumor biopsies from a clinical phase I/II study in which nadunolimab was administered to patients.
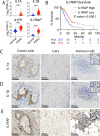
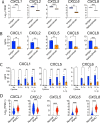
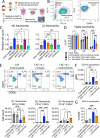
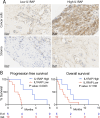
Results: In the xenograft mouse model, nadunolimab exhibited antitumor effects only when human CAFs were co-transplanted with PDAC cells. IL-1 stimulation induced CAFs to secrete chemokines that recruited neutrophils and monocytes. The secretion of this chemokine and the migration of myeloid cells were inhibited by nadunolimab. Media conditioned by IL-1-stimulated CAFs sustained a neutrophil population with a tissue invasion phenotype, an effect that was reversed by nadunolimab. In a cohort of metastatic late-stage PDAC patients receiving nadunolimab as monotherapy, high IL1RAP expression in tumors was associated with extended progression-free survival.
Conclusions: Our study demonstrates that targeting IL1RAP on CAFs inhibits IL-1-induced chemokine secretion and recruitment of neutrophils and monocytes, thereby counteracting the immunosuppressive microenvironment in PDAC. These findings highlight the therapeutic potential of targeting IL1RAP in PDAC.
Keywords: Cytokine; Monoclonal antibody; Monocyte; Neutrophil.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NH, PS, CG, SM, KvW, CRM, DL, and MJ are current employees of, and/or hold stocks or stock options in Cantargia AB, Lund, Sweden. The use of nadunolimab for cancer treatment is patented within WO 2015/13602.
Figures

References
-
- Biffi G, Oni TE, Spielman B, et al. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019;9:282–301. doi: 10.1158/2159-8290.CD-18-0710. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
