Inhibition of Pyroptosis by Hydroxychloroquine as a Neuroprotective Strategy in Ischemic Stroke
- PMID: 39694827
- PMCID: PMC11728853
- DOI: 10.1523/ENEURO.0254-24.2024
Inhibition of Pyroptosis by Hydroxychloroquine as a Neuroprotective Strategy in Ischemic Stroke
Abstract
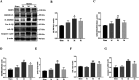
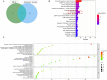
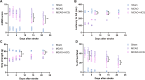
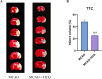
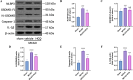
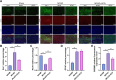
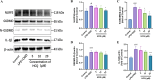
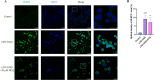
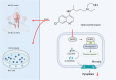
Hydroxychloroquine (HCQ), a well-known antimalarial and anti-inflammatory drug, has demonstrated potential neuroprotective effects in ischemic stroke by inhibiting pyroptosis, a programmed cell death associated with inflammation. This study investigates the impact of HCQ on ischemic stroke pathology using both in vivo and in vitro models. In vivo, C57BL/6 mice subjected to middle cerebral artery occlusion (MCAO) were treated with HCQ. Neurological deficits, infarct volume, and the expression of pyroptosis markers were evaluated. The results demonstrated that HCQ significantly improved motor function and reduced infarct volume in the MCAO mouse model. In vitro, BV2 microglial cells exposed to lipopolysaccharide (LPS) and oxygen-glucose deprivation (OGD) were treated with HCQ. Western blot and immunofluorescence analyses revealed that HCQ effectively suppressed the expression of pyroptosis markers GSDMD and NLRP3 in both in vivo and in vitro models. These findings suggest that HCQ mitigates ischemic stroke damage by inhibiting pyroptosis, highlighting its potential as a therapeutic agent for ischemic stroke. This study provides novel insights into the molecular mechanisms by which HCQ exerts its neuroprotective effects, offering a promising new avenue for developing safe, cost-effective, and widely applicable stroke treatments. The potential of HCQ to modulate neuroinflammatory pathways presents a significant advancement in ischemic stroke therapy, emphasizing the importance of targeting pyroptosis in stroke management and the broader implications for treating neuroinflammatory conditions.Significance Statement Ischemic stroke remains a leading cause of disability and death globally, with limited effective treatments. This study reveals that HCQ significantly mitigates ischemic stroke damage by inhibiting pyroptosis, a form of programmed cell death. Using in vivo and in vitro models, HCQ was shown to improve motor function and reduce infarct volume, highlighting its potential as a neuroprotective agent. These findings offer a promising new therapeutic approach for ischemic stroke, emphasizing the importance of targeting pyroptosis in stroke treatment.
Copyright © 2024 Peng et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
LinkOut - more resources
Full Text Sources
